CIPRO Film-coated tablet / Oral suspension kit Ref.[10545] Active ingredients: Ciprofloxacin
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
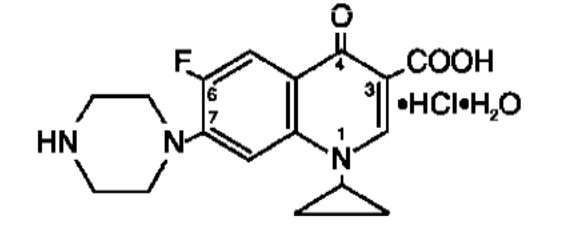
CIPRO (ciprofloxacin hydrochloride) Tablets and CIPRO (ciprofloxacin) Oral Suspension are synthetic antimicrobial agents for oral administration. Ciprofloxacin hydrochloride, USP, a fluoroquinolone, is the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1, 4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. It is a faintly yellowish to light yellow crystalline substance with a molecular weight of 385.8.
Its empirical formula is C17H18FN3O3•HCl•H2O and its chemical structure is as follows:
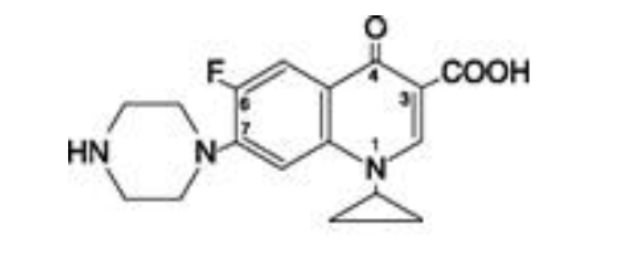
Ciprofloxacin is 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic acid. Its empirical formula is C17H18FN3O3 and its molecular weight is 331.4.
It is a faintly yellowish to light yellow crystalline substance and its chemical structure is as follows:
CIPRO film-coated tablets are available in 250 mg and 500 mg (ciprofloxacin equivalent) strengths. Each CIPRO film-coated tablet contains 250 mg (equivalent to 291 mg ciprofloxacin hydrochloride monohydrate) or 500 mg of ciprofloxacin (equivalent to 582 mg ciprofloxacin hydrochloride monohydrate) CIPRO tablets are white to slightly yellowish. The inactive ingredients are cornstarch, crospovidone, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, and titanium dioxide.
CIPRO Oral Suspension is available in 5% (5 g ciprofloxacin in 100 mL) and 10% (10 g ciprofloxacin in 100 mL) strengths. CIPRO Oral Suspension is a white to slightly yellowish suspension with strawberry flavor which may contain yellow-orange droplets. It is composed of ciprofloxacin microcapsules and diluent which are mixed prior to dispensing [see Dosage and Administration (2.5)].
The components of the suspension have the following compositions:
- Microcapsules–ciprofloxacin, hypromellose, magnesium stearate, methacrylic acid copolymer, Polysorbate 20 and povidone.
- Diluent–medium-chain triglycerides, sucrose, soy-lecithin, water, and strawberry flavor.
- Five (5) mL of 5% suspension contains approximately 1.4 g of sucrose and 5 mL of 10% suspension contains approximately 1.3 g of sucrose.
| Dosage Forms and Strengths |
|---|
3.1 Tablets
3.2 Oral Suspension
|
| How Supplied | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CIPRO (ciprofloxacin hydrochloride) Tablets are available as round, slightly yellowish film-coated tablets containing 250 mg ciprofloxacin. The 250 mg tablet is coded with the word "BAYER" on one side and "CIP 250" on the reverse side. CIPRO is also available as capsule shaped, slightly yellowish film-coated tablets containing 500 mg ciprofloxacin. The 500 mg tablet is coded with the word "BAYER" on one side and "CIP 500" on the reverse side. CIPRO 250 mg and 500 mg are available in bottles of 100.
CIPRO Oral Suspension is supplied in 5% and 10% strengths. The drug product is composed of two components (microcapsules containing the active ingredient and diluent) which must be mixed by the pharmacist [see Dosage and Administration (2.5)].
|
Drugs
| Drug | Countries | |
|---|---|---|
| CIPRO | Albania, Canada, Germany, Singapore, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.