CLINIMIX E Solution for injection Ref.[50762] Active ingredients: Alanine Arginine Calcium chloride Glucose Glycine Histidine Isoleucine Leucine Lysine Magnesium chloride Methionine Phenylalanine Potassium phosphate Proline Serine Sodium acetate Sodium chloride Threonine Tryptophan Tyrosine Valine
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
CLINIMIX E sulfite-free (amino acids with electrolytes in dextrose with calcium) injection for intravenous use consists of sterile, nonpyrogenic, hypertonic solutions in a dual chamber container.
The outlet port chamber contains essential and nonessential amino acids with electrolytes. The formulas for the individual electrolytes and amino acids are provided in Table 8.
Table 8. Formulas for Electrolytes and Amino Acids:
| Electrolytes | |
| Sodium Acetate | C2H3NaO2•3H2O |
| Potassium Phosphate, dibasic | K2HPO4 |
| Magnesium Chloride | MgCl2•6H2O |
| Sodium Chloride | NaCl |
| Essential Amino Acids | |
| Leucine | (CH3)2 CHCH2CH (NH2) COOH |
| Isoleucine | CH3CH2CH (CH3) CH (NH2) COOH |
| Valine | (CH3)2 CHCH (NH2) COOH |
| Lysine (added as the hydrochloride salt) | H2N (CH2)4 CH (NH2) COOH |
| Phenylalanine | (C6H5) CH2 CH (NH2) COOH |
| Histidine | (C3H3N2) CH2CH (NH2) COOH |
| Threonine | CH3CH (OH) CH (NH2) COO |
| Methionine | CH3S (CH2)2 CH (NH2) COOH |
| Tryptophan | (C8H6N) CH2 CH (NH2) COOH |
| Nonessential Amino Acids | |
| Alanine | CH3CH (NH2) COOH |
| Arginine | H2NC (NH) NH (CH2)3 CH (NH2) COOH |
| Glycine | H2NCH2COOH |
| Proline | [(CH2)3 NH CH] COOH |
| Serine | HOCH2CH (NH2) COOH |
| Tyrosine | [C6H4 (OH)] CH2CH (NH2) COOH |
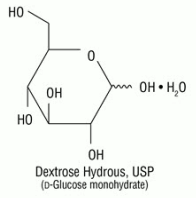
The injection port chamber contains dextrose with calcium. The formula for Calcium Chloride is: CaCl2•2H2O. Dextrose, USP, is chemically designated D-glucose, monohydrate (C6H12O6 • H2O) and has the following structure:
Dextrose is derived from corn.
See Table 7 for composition, pH, osmolarity, ionic concentration and caloric content of the admixed product [see Dosage Forms and Strengths (3)].
The dual chamber container is a lipid-compatible plastic container (PL 2401 Plastic).
CLINIMIX E contains no more than 25 mcg/L of aluminum.
| Dosage Forms and Strengths | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CLINIMIX E injection is available in 1000 mL and 2000 mL dual chamber containers. The individual chambers contain essential and nonessential amino acids with electrolytes and dextrose with calcium. Table 7 describes the individual components of CLINIMIX E. Table 7. INGREDIENTS PER 100mL OF CLINIMIX E:
* Balanced by ions from amino acids. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| How Supplied | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
CLINIMIX E (amino acids with electrolytes in dextrose with calcium) injection (sulfite-free) is available in 1000 mL and 2000 mL volumes (See Table 9). Table 9. CLINIMIX E Formulations:
Baxter Healthcare Corporation, Deerfield, IL 60015, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| CLINIMIX E | Canada, Finland, France, Poland, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.