COGESIC Tablet Ref.[50555] Active ingredients: Codeine Paracetamol
Source: Marketing Authorisation Holder Revision Year: 2022 Publisher: Oethmaan Biosims (PTY) Ltd, Office 207A, Sherwood House, Greenacres Office Park, c/o Victory and Rustenburg Roads, Victory Park, Johannesburg 2195
4.1. Therapeutic indications
COGESIC tablets are indicated for the relief of mild to moderate pain and for the reduction of temperature in febrile conditions.
4.2. Posology and method of administration
DO NOT EXCEED THE RECOMMENDED DOSE.
Adults
One or two tablets every four to six hours. Do not exceed an adult dose of 8 tablets per day.
Children over 12 years
One tablet every four to Or hours.
Children 6 to 12 years
Half to one tablet every six hours.
Do not use continuously for longer than five (5) days without consulting your doctor (see “WARNINGS AND SPECIAL PRECAUTIONS”).
4.9. Overdose
Paracetamol
Prompt treatment Is essential.
In the event of an overdosage, consult a doctor immediately, or take the person to a hospital directly. A delay in starting treatment may mean that the antidote is given too late to be effective. Evidence of liver damage is often delayed until after the time for effective treatment has lapsed.
Susceptibility to paracetamol toxicity is increased in patients who have taken repeated high doses (greater than 5-10 g/day) of paracetamol for several days, in chronic alcoholism, chronic liver disease, AIDS, malnutrition and with the use of medicines that include liver microsomal oxidation such as barbiturates, isoniazid, rifampicin, phenytoin and carbamarepine.
Symptoms of paracetamol overdosage in the first 24 hours include pallor, nausea, vomOng, anomie, and possibly abdominal pain. Mild symptoms during the first two days of acute poisoning do not reflect the potential seriousness of the overdosage. Liver damage may become apparent 12 to 48 hours, or later after ingestion, initially by elevation of the serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentration and prolongation of prothrombin time. The liver damage may lead to encephalopathy, coma and death.
Acute renal failure with acute tubular necrosis may develop even in the absence of severe liver damage. Abnormalities of glucose metabolism and metabolic acidosis may occur. Cardiac arrhylhmias have been reported.
Treatment for paracetamol overdosage
Although evidence is limited it is recommended that any adult person who has ingested about 5-10 grams or more of paracetamol (or a child who has had more than 140 mg/kg) within the preceding four hours should have the stomach emptied by lavage (emesis may be adequate for children) and a single dose of 50 g activated charcoal given via the lavage tube. Ingestion of amounts of paracetamol smaller than this may require treatment in patients susceptible to paracetamol poisoning (see above). In patients who are stuperose or comatose, endotracheal intubation should precede gastric lavage in order to avoid aspiration.
N-acetylcysteine should be administered to all cases of suspected overdosage as soon as possible, preferably within 8 hours of overdosage, although treatment up to 36 hours after ingestion may still be of benefit, especially if more than 150 mg/kg of paracetamol was taken. An initial dose of 150 mg/kg N-acetylcysteine in 200 ml dextrose injection given intravenously over 15 minutes, followed by an infusion of 50 mg/kg in 500 ml dextrose injection over the next 4 hours, and then 100 mg/kg in 1000 ml dextrose injection over the next 16 hours. The volume of intravenous fluids should be modified for children.
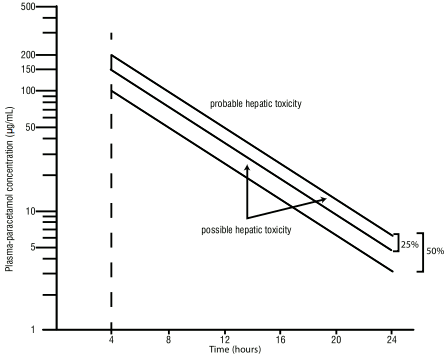
Although the oral formulation is not the treatment of choice, 140 mg/kg dissolved in water may be administered initially, followed by 70 mg/kg every 4 hours for 17 doses. A plasma paracetamol level should be determined 4 hours after ingestion in all cases of suspected overdosage. Levels done before 4 hours, unless high, may be misleading. Patients at risk of Neer damage, and hence requiring continued treatment with N-acetylcysteine, can be identified according to their plasma paracetamol level. The plasma paracetamol level can be plotted against time since ingestion in the nomogram below. The nomogram should be used only in relation to a single acute ingestion.
Those whose plasma paracetamol levels are above the “normal treatment line”, should continue N-acetylcysteine treatment with 100 mg/kg IV over 16 hours repeatedly until recovery. Patients with increased susceptibility to liver damage as identified above, should continue treatment if concentrations are above the “high risk treatment line”. Prothrombin index correlates best with survival.
Monitor all patients with significant ingestion for at least ninety six hours.
Figure 1. A semi- logarithmic plot of plasma-paracetamol concentration against hours after ingestion:
Sweetman, S ed. (2002) ‘Martindale The Complete Drug Reference’ Great Britain, The Bath Press (pg 72)
The latest information regarding the treatment of overdosage can be obtained from the nearest poison information centre.
Codeine
Symptoms include restlessness, excitement, respiratory depression and hypotension with circulatory failure and coma. In children convulsions may occur. The specific antagonist, naffixone hydrochloride is used to counteract the severe respiratory depression.
In the event of overdosage, consult a doctor or take the patient to the nearest hospital immediately.
Treatment is supportive and symptomatic.
6.4. Special precautions for storage
Store at or below 25°C and protect from strong light in a well-closed container. Protect from moisture. Exposure to air should be minimal.
KEEP OUT OF REACH OF CHILDREN.
6.5. Nature and contents of container
Cartons containing 2 × 10 tablets in push through blister packs.
White plastic (LOPE) Ziploc bag (Patient Ready Pack) containing 56 tablets.
Amber plastic (PVC) bottles containing 100, 500, and 1000 tablets.
White plastic (HOPE) bottles containing 1000 tablets.
Blue/green plastic buckets containing 5000 tablets.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.