COVERAM Tablet Ref.[50881] Active ingredients: Amlodipine Perindopril
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2022 Publisher: Servier Laboratories (Aust.) Pty Ltd, www.servier.com.au, Level 4, Building 9, 588A Swan Street, Burnley, 3121, Victoria
4.1. Therapeutic indications
COVERAM is indicated as substitution therapy for the treatment of hypertension and/or stable coronary heart disease in patients already controlled with separate doses of perindopril and amlodipine, given concurrently at the same dose level. Treatment should not be initiated with this combination.
4.2. Posology and method of administration
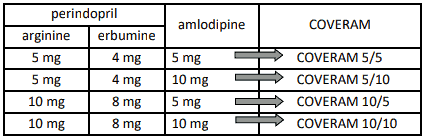
COVERAM (perindopril arginine/amlodipine) is available in strengths of 5 mg/5 mg, 5 mg/10 mg, 10 mg/5 mg and 10 mg/10 mg as substitution therapy for patients already controlled with separate doses of perindopril (5 or 10 mg) and amlodipine (5 or 10 mg), given concurrently at the dose level as indicated in the table below. Treatment should not be initiated with this combination.
Table 1. Dose conversion from perindopril and amlodipine to COVERAM:
Food intake may reduce hepatic biotransformation of perindopril to perindoprilat. Recommended treatment is one tablet per day as a single dose, preferably to be taken in the morning and before a meal.
As perindopril and amlodipine may be used for different clinical indications, dose adjustments should be based on clinical judgment and the individual patient profile.
Adjustments can be made by decreasing or increasing the dose of either perindopril and/or amlodipine using separate perindopril and/or amlodipine products within the recommended dose range until clinical stability is re-established. Consult the Product Information of the individual perindopril and/or amlodipine products being used when adjusting the dose.
In the event that down-titration is required, adjustments using amlodipine 2.5 mg or a dose of perindopril equivalent to perindopril arginine 2.5 mg, as separate products should be considered until clinical stability is re-established.
Patients with impaired renal function and elderly patients
Elimination of perindoprilat is decreased in the elderly and in patients with renal failure. Therefore, the usual medical follow-up will include frequent monitoring of creatinine and potassium.
Where down-titration is required to achieve clinical stability in patients with a CrCl <60mL/min, adjustments using amlodipine 2.5 mg or a dose of perindopril equivalent to perindopril arginine 2.5 mg, as separate products should be considered until clinical stability is re-established. Please consult the product information of the individual perindopril or amlodipine products.
Changes in amlodipine plasma concentrations are not correlated with degree of renal impairment.
Amlodipine is not dialysable.
In elderly patients, adjustments using amlodipine and perindopril as separate products should be considered. Small, fragile or elderly individuals should be started on amlodipine 2.5 mg once daily and care should be taken when increasing the dosage of amlodipine. The initial dose of perindopril in the elderly should always be a dose equivalent to perindopril arginine 2.5 mg daily and patients should be monitored closely during the initial stages of treatment.
Patients with impaired hepatic function
Dosage recommendations have not been established in patients with mild to moderate hepatic impairment therefore COVERAM should be administered with caution and treatment should start at the lower end of the dosing range (see section 4.4 - Special warnings and precautions for use and section 5.2 - Pharmacokinetic properties). Dose adjustments can be made by decreasing or increasing the dose of either perindopril and/or amlodipine using separate perindopril and/or amlodipine products within the recommended dose range until an optimal starting and maintenance dose is found. Patients with hepatic impairment should be started on amlodipine 2.5 mg once daily.
4.9. Overdose
For information on the management of overdose, contact the Poison Information Centre on 131126 (Australia).
There is no information on overdosage with COVERAM in humans.
Related to perindopril component
Limited data are available for overdosage in humans. Symptoms associated with overdosage of ACE inhibitors may include hypotension, circulatory shock, electrolyte disturbances, renal failure, hyperventilation, tachycardia, palpitations, bradycardia, dizziness, anxiety, and cough. The recommended treatment of overdosage is intravenous infusion of normal saline solution. If hypotension occurs, the patient should be placed in the shock position. If available, treatment with Angiotensin II infusion and/or intravenous catecholamines may also be considered. Perindopril may be removed from the general circulation by haemodialysis (see section 4.4 - Special warnings and precautions for use). Vital signs, serum electrolytes and creatinine concentrations should be monitored continuously. Pacemaker therapy is indicated for treatment resistant bradycardia.
Related to amlodipine component
Available data suggest that overdosage might be expected to cause excessive peripheral vasodilation with marked hypotension and possibly a reflex tachycardia. Dysrhythmias may occur following overdose with any calcium antagonists. Hypotension and bradycardia are usually seen within one to five hours following overdose. Hypotension can persist for longer than 24 hours despite treatment. Cardiac rhythm disturbances have been noted to persist for up to seven days. Marked and probably prolonged systemic hypotension up to and including shock with fatal outcome have been reported.
Rarely, non-cardiogenic pulmonary oedema has been reported as a consequence of amlodipine overdose, that may manifest with a delayed onset (24-48 hours post-ingestion) and require ventilatory support. Early resuscitative measures (including fluid overload) to maintain perfusion and cardiac output may be precipitating factors.
If massive overdose should occur, active cardiac and respiratory monitoring should be instituted. Frequent blood pressure measurements are essential. Should hypotension occur, cardiovascular support including elevation of the extremities and the judicious administration of fluids should be initiated. If hypotension remains unresponsive to these conservative measures, administration of vasopressors (such as phenylephrine), should be considered with attention to circulating volume and urine output. Intravenous calcium may help to reverse the effects of calcium entry blockade. Administration of activated charcoal to healthy volunteers immediately or up to two hours after ingestion of amlodipine 10 mg has been shown to significantly decrease amlodipine absorption. In patients who are not fully conscious or have impaired gag reflex, consideration should be given to administering activated charcoal via nasogastric tube once the airway is protected. Ipecac-emesis is not recommended since haemodynamic instability and CNS depression may rapidly develop. Since amlodipine is highly protein-bound, dialysis is not likely to be of benefit.
6.3. Shelf life
3 years.
6.4. Special precautions for storage
Store in a dry place below 25°C. Keep the container tightly closed and protect from light.
6.5. Nature and contents of container
Thirty (30) tablets supplied in a white HDPE bottle equipped with a white induction-sealed child resistantclosure and desiccant sachets. COVERAM 5/5 only, is also supplied in a 10-tablet bottle.
6.6. Special precautions for disposal and other handling
In Australia, any unused medicine or waste material should be disposed of by taking it to your local pharmacy.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.