DIOVAN HCT Film-coated tablet Ref.[10573] Active ingredients: Hydrochlorothiazide Valsartan
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Diovan HCT (valsartan and hydrochlorothiazide, USP) is a combination of valsartan, an orally active, specific angiotensin II receptor blocker (ARB) acting on the AT1 receptor subtype, and hydrochlorothiazide, a diuretic.
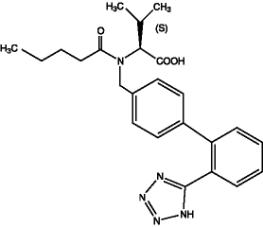
Valsartan, a nonpeptide molecule, is chemically described as N-(1-oxopentyl)- N-[[2′-(1H-tetrazol-5-yl)[1,1′-biphenyl]-4-yl]methyl]-L-Valine. Its empirical formula is C24H29N5O3, its molecular weight is 435.5, and its structural formula is:
Valsartan is a white to practically white fine powder. It is soluble in ethanol and methanol and slightly soluble in water.
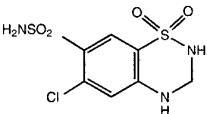
Hydrochlorothiazide, USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water; freely soluble in sodium hydroxide solution, in n-butylamine, and in dimethylformamide; sparingly soluble in methanol; and insoluble in ether, in chloroform, and in dilute mineral acids. Hydrochlorothiazide is chemically described as 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide.
Hydrochlorothiazide is a thiazide diuretic. Its empirical formula is C7H8ClN3O4S2, its molecular weight is 297.73, and its structural formula is:
Diovan HCT tablets are formulated for oral administration to contain valsartan and hydrochlorothiazide, USP 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg, and 320/25 mg. The inactive ingredients of the tablets are colloidal silicon dioxide, crospovidone, hydroxypropyl methylcellulose, iron oxides, magnesium stearate, microcrystalline cellulose, polyethylene glycol, talc, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
80/12.5 mg tablets, imprinted CG/HGH (Side 1/Side 2). 160/12.5 mg tablets, imprinted CG/HHH. 160/25 mg tablets, imprinted NVR/HXH. 320/12.5 mg tablets, imprinted NVR/HIL. 320/25 mg tablets, imprinted NVR/CTI. |
| How Supplied |
|---|
|
Diovan HCT (valsartan and hydrochlorothiazide, USP) is available as non-scored tablets containing valsartan/hydrochlorothiazide 80/12.5 mg, 160/12.5 mg, 160/25 mg, 320/12.5 mg, and 320/25 mg. Strengths are available as follows. 80/12.5 mg Tablet: Light orange, ovaloid, with slightly convex faces debossed CG on 1 side and HGH on the other side. Bottles of 90 – NDC 0078-0314-34 160/12.5 mg Tablet: Dark red, ovaloid, with slightly convex faces debossed CG on 1 side and HHH on the other side. Bottles of 90 – NDC 0078-0315-34 160/25 mg Tablet: Brown orange, ovaloid, with slightly convex faces debossed NVR on 1 side and HXH on the other side. Bottles of 90 – NDC 0078-0383-34 320/12.5 mg Tablet: Pink, ovaloid, with beveled edge, debossed NVR on 1 side and HIL on the other side. Bottles of 90 – NDC 0078-0471-34 320/25 mg Tablet: Yellow, ovaloid, with beveled edge, debossed NVR on 1 side and CTI on the other side. Bottles of 90 – NDC 0078-0472-34 |
Drugs
| Drug | Countries | |
|---|---|---|
| DIOVAN HCT | Brazil, Canada, Ecuador, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.