DIOVAN HCT Film-coated tablet Ref.[10573] Active ingredients: Hydrochlorothiazide Valsartan
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
Diovan HCT (valsartan and hydrochlorothiazide, USP) is indicated for the treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes, including hydrochlorothiazide and the angiotensin II receptor blocker (ARB) class to which valsartan principally belongs. There are no controlled trials demonstrating risk reduction with Diovan HCT.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality have also been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (e.g., patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
Add-On Therapy
Diovan HCT may be used in patients whose blood pressure is not adequately controlled on monotherapy.
Replacement Therapy
Diovan HCT may be substituted for the titrated components.
Initial Therapy
Diovan HCT may be used as initial therapy in patients who are likely to need multiple drugs to achieve blood pressure goals.
The choice of Diovan HCT as initial therapy for hypertension should be based on an assessment of potential benefits and risks.
Patients with stage 2 hypertension are at a relatively high risk for cardiovascular events (such as strokes, heart attacks, and heart failure), kidney failure, and vision problems, so prompt treatment is clinically relevant. The decision to use a combination as initial therapy should be individualized and should be shaped by considerations such as baseline blood pressure, the target goal, and the incremental likelihood of achieving goal with a combination compared to monotherapy. Individual blood pressure goals may vary based upon the patient's risk.
Data from the high dose multifactorial trial [see Clinical Studies (14.1)] provides estimates of the probability of reaching a target blood pressure with Diovan HCT compared to valsartan or hydrochlorothiazide monotherapy. The figures below provide estimates of the likelihood of achieving systolic or diastolic blood pressure control with Diovan HCT 320/25 mg, based upon baseline systolic or diastolic blood pressure. The curve of each treatment group was estimated by logistic regression modeling. The estimated likelihood at the right tail of each curve is less reliable due to small numbers of subjects with high baseline blood pressures.
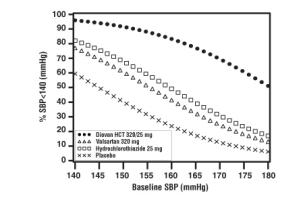 Figure 1: Probability of Achieving Systolic Blood Pressure < 140 mmHg at Week 8 | 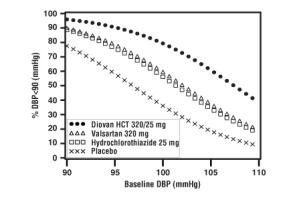 Figure 2: Probability of Achieving Diastolic Blood Pressure < 90 mmHg at Week 8 |
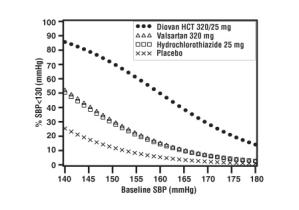 Figure 3: Probability of Achieving Systolic Blood Pressure < 130 mmHg at Week 8 | 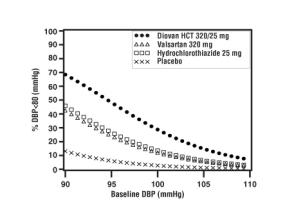 Figure 4: Probability of Achieving Diastolic Blood Pressure < 80 mmHg at Week 8 |
For example, a patient with a baseline blood pressure of 160/100 mmHg has about a 41% likelihood of achieving a goal of <140 mmHg (systolic) and 60% likelihood of achieving <90 mmHg (diastolic) on valsartan alone and the likelihood of achieving these goals on HCTZ alone is about 50% (systolic) or 57% (diastolic). The likelihood of achieving these goals on Diovan HCT rises to about 84% (systolic) or 80% (diastolic). The likelihood of achieving these goals on placebo is about 23% (systolic) or 36% (diastolic).
2. Dosage and Administration
2.1 General Considerations
The usual starting dose is Diovan HCT 160/12.5 mg once daily. The dosage can be increased after 1 to 2 weeks of therapy to a maximum of one 320/25 tablet once daily as needed to control blood pressure [see Clinical Studies (14.2)]. Maximum antihypertensive effects are attained within 2 to 4 weeks after a change in dose.
2.2 Add-On Therapy
A patient whose blood pressure is not adequately controlled with valsartan (or another ARB) alone or hydrochlorothiazide alone may be switched to combination therapy with Diovan HCT.
A patient who experiences dose-limiting adverse reactions on either component alone may be switched to Diovan HCT containing a lower dose of that component in combination with the other to achieve similar blood pressure reductions. The clinical response to Diovan HCT should be subsequently evaluated and if blood pressure remains uncontrolled after 3 to 4 weeks of therapy, the dose may be titrated up to a maximum of 320/25 mg.
2.3 Replacement Therapy
Diovan HCT may be substituted for the titrated components.
2.4 Initial Therapy
Diovan HCT is not recommended as initial therapy in patients with intravascular volume depletion [see Warnings and Precautions (5.2)].
2.5 Use with Other Antihypertensive Drugs
Diovan HCT may be administered with other antihypertensive agents.
10. Overdosage
Limited data are available related to overdosage in humans. The most likely manifestations of overdosage would be hypotension and tachycardia; bradycardia could occur from parasympathetic (vagal) stimulation. Depressed level of consciousness, circulatory collapse and shock have been reported. If symptomatic hypotension should occur institute supportive treatment.
Valsartan is not removed from the plasma by dialysis.
The degree to which hydrochlorothiazide is removed by hemodialysis has not been established. The most common signs and symptoms observed in patients are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias.
In rats and marmosets, single oral doses of valsartan up to 1524 and 762 mg/kg in combination with hydrochlorothiazide at doses up to 476 and 238 mg/kg, respectively, did not show any adverse treatment-related effects. These no adverse effect doses in rats and marmosets, respectively, represent 46.5 and 23 times the MRHD of valsartan and 188 and 113 times the MRHD of hydrochlorothiazide on a mg/m2 basis (Calculations assume an oral dose of 320 mg/day valsartan in combination with 25 mg/day hydrochlorothiazide and a 60-kg patient).
Valsartan was without grossly observable adverse effects at single oral doses up to 2,000 mg/kg in rats and up to 1,000 mg/kg in marmosets, except for salivation and diarrhea in the rat and vomiting in the marmoset at the highest dose (60 and 31 times, respectively, the MRHD on a mg/m2 basis) (Calculations assume an oral dose of 320 mg/day and a 60-kg patient).
The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in both mice and rats, which represents 2027 and 4054 times, respectively, the MRHD on a mg/m2 basis (Calculations assume an oral dose of 25 mg/day and a 60-kg patient).
16.2. Storage and Handling
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.