EDARBYCLOR Tablet Ref.[10008] Active ingredients: Azilsartan medoxomil Chlortalidone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Edarbyclor is a combination of azilsartan medoxomil (angiotensin II receptor blocker; as its potassium salt) and chlorthalidone (thiazide-like diuretic).
Azilsartan medoxomil, a prodrug, is hydrolyzed to azilsartan in the gastrointestinal tract during absorption. Azilsartan is an angiotensin II receptor blocker. Chlorthalidone is a monosulfamyl thiazide-like diuretic that differs chemically from thiazide diuretics by the lack of a benzothiadiazine structure.
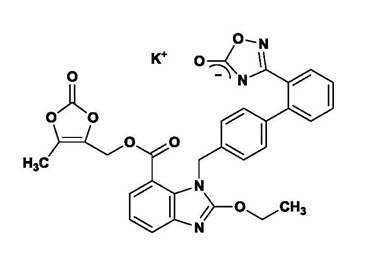
The potassium salt of azilsartan medoxomil, azilsartan kamedoxomil, is chemically described as (5-Methyl-2-oxo-1,3-dioxol-4-yl)methyl 2-ethoxy-1-{[2'-(5-oxo-4,5-dihydro-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]methyl}-1H-benzimidazole-7-carboxylate monopotassium salt. Its empirical formula is C30H23KN4O8.
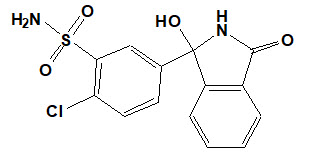
Chlorthalidone is chemically described as 2-chloro-5(1-hydroxy-3-oxo-1- isoindolinyl) benzenesulfonamide. Its empirical formula is C14H11ClN2O4S.
The structural formula for azilsartan medoxomil is:
The structural formula for chlorthalidone is:
Azilsartan kamedoxomil is a white to nearly white powder with a molecular weight of 606.62. It is practically insoluble in water and freely soluble in methanol.
Chlorthalidone is a white to yellowish white powder with a molecular weight of 338.76. Chlorthalidone is practically insoluble in water, in ether, and in chloroform; soluble in methanol; slightly soluble in ethanol.
Edarbyclor is available for oral use as tablets. The tablets have a characteristic odor. Each Edarbyclor tablet contains 42.68 mg of azilsartan kamedoxomil, which is equivalent to containing azilsartan medoxomil 40 mg plus 12.5 or 25 mg of chlorthalidone. Each tablet of Edarbyclor also contains the following inactive ingredients: mannitol, microcrystalline cellulose, fumaric acid, sodium hydroxide, hydroxypropyl cellulose, crospovidone, magnesium stearate, hypromellose 2910, talc, titanium dioxide, ferric oxide red, polyethylene glycol 8000, and printing ink gray F1.
| Dosage Forms and Strengths |
|---|
|
Edarbyclor is supplied in the following dosage strengths:
|
| How Supplied | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Edarbyclor is supplied as fixed dose combination tablets that are round, biconvex, film-coated, and 9.7 mm in diameter.
|
Drugs
| Drug | Countries | |
|---|---|---|
| EDARBYCLOR | Canada, Hong Kong, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.