ENTEREG Capsule Ref.[9957] Active ingredients: Alvimopan
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
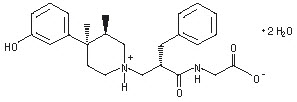
ENTEREG capsules contain alvimopan, an opioid antagonist. Chemically, alvimopan is the single stereoisomer [[2(S)[[4(R)(3-hydroxyphenyl)-3(R),4-dimethyl-1-piperidinyl]methyl]-1-oxo-3-phenylpropyl]amino]acetic acid dihydrate. It has the following structural formula:
Alvimopan is a white to light beige powder with a molecular weight of 460.6, and the empirical formula is C25H32N2O4∙2H2O. It has a solubility of <0.1 mg/mL in water or buffered solutions between pH 3.0 and 9.0, 1 to 5 mg/mL in buffered solutions at pH 1.2, and 10 to 25 mg/mL in aqueous 0.1 N sodium hydroxide. At physiological pH, alvimopan is zwitterionic, a property that contributes to its low solubility.
ENTEREG capsules for oral administration contain 12 mg of alvimopan on an anhydrous basis suspended in the inactive ingredient polyethylene glycol.
| Dosage Forms and Strengths |
|---|
|
12 mg blue, hard-gelatin capsules with “ADL2698” printed on both the body and the cap of the capsule. |
| How Supplied |
|---|
|
ENTEREG capsules, 12 mg, are blue, hard-gelatin capsules printed with “ADL2698” on both the body and the cap of the capsule. ENTEREG capsules are available in unit-dose packs of 30 capsules (30 doses) (NDC 67919-020-10) for hospital use only. |
Drugs
| Drug | Countries | |
|---|---|---|
| ENTEREG | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.