EZETAST Film-coated tablet Ref.[49962] Active ingredients: Atorvastatin Ezetimibe
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2021 Publisher: Lupin Australia Pty Ltd, Suite 2, Level 2, 19-23 Prospect Street, Box Hill, VIC, 3128 Distributor: Arrotex Pharmaceuticals Pty Ltd, 15-17 Chapel Street, Cremorne, VIC, 3121, Australia
Product name and form
Ezetimibe.
Atorvastatin (as calcium trihydrate).
| Pharmaceutical Form |
|---|
|
EZETAST 10/10: White to off-white, capsule-shaped, biconvex, film-coated tablet debossed with 'AE' on one side and '1' on the other side. EZETAST 10/20: White to off-white, capsule-shaped, biconvex, film-coated tablet debossed with 'AE' on one side and '2' on the other side. EZETAST 10/40: White to off-white, capsule-shaped, biconvex, film-coated tablet debossed with 'AE' on one side and '3' on the other side. EZETAST 10/80: White to off-white, capsule-shaped, biconvex, film-coated tablet debossed with 'AE' on one side and '4' on the other side. |
Qualitative and quantitative composition
EZETAST is a film-coated tablet containing ezetimibe 10 mg and atorvastatin 10, 20, 40 or 80 mg.
EZETAST 10/10: Each tablet contains 10 mg of ezetimibe and atorvastatin calcium trihydrate equivalent to 10 mg of atorvastatin.
EZETAST 10/20: Each tablet contains 10 mg of ezetimibe and atorvastatin calcium trihydrate equivalent to 20 mg of atorvastatin.
EZETAST 10/40: Each tablet contains 10 mg of ezetimibe and atorvastatin calcium trihydrate equivalent to 40 mg of atorvastatin.
EZETAST 10/80: Each tablet contains 10 mg of ezetimibe and atorvastatin calcium trihydrate equivalent to 80 mg of atorvastatin.
Excipients with known effect: contains sugars as lactose.
For the full list of excipients, see Section 6.1 List of Excipients.
Physicochemical Properties
Chemical Structure
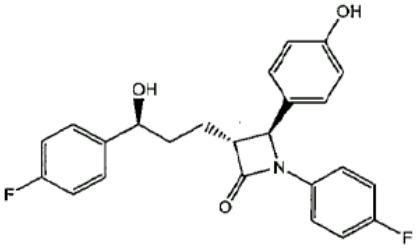
Ezetimibe
Ezetimibe is a white to off-white, crystalline powder that is freely soluble in acetonitrile, insoluble in aqueous solvents and non-polar solvents like hexane.
Chemical Name: 1-(4-fluorophenyl)-3==(R)==-[3-(4-fluorophenyl)-3(S)hydroxypropyl]-4(S)-(4-hydroxyphenyl)-2-azetidinone
Molecular Formula: C24H21F2NO3
Molecular Weight: 409.43
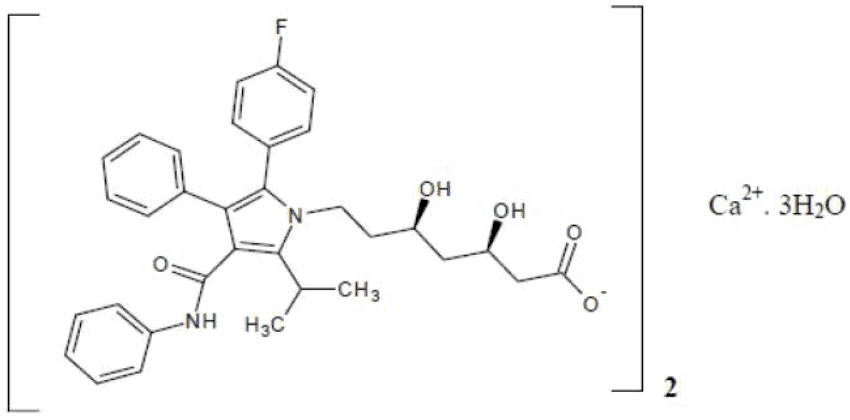
Atorvastatin calcium trihydrate
Atorvastatin calcium trihydrate is a white to off-white crystalline powder that is sparingly soluble in methanol.
Chemical Name: [R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dihydroxy-5-(1-methylethyl)-3-phenyl4[(phenylamino) carbonyl]-1H-pyrrole -1-heptanoic acid, calcium salt (2:1) trihydrate
Molecular Formula: C66H68CaF2N4O10.3H2O
Molecular Weight: 1209.42
CAS Number
Ezetimibe: 163222-33-1
Atorvastatin: 344423-98-9
| Active Ingredient |
|---|
|
Atorvastatin is a selective, competitive inhibitor of HMG-CoA reductase. Atorvastatin lowers plasma cholesterol and lipoprotein serum concentrations by inhibiting HMG-CoA reductase and subsequently cholesterol biosynthesis in the liver and increases the number of hepatic LDL receptors on the cell surface for enhanced uptake and catabolism of LDL. |
|
Ezetimibe is in a new class of lipid-lowering compounds that selectively inhibit the intestinal absorption of cholesterol. The molecular target of ezetimibe is the sterol transporter, Niemann-Pick C1-Like 1 (NPC1L1), which is responsible for the intestinal uptake of cholesterol. |
| List of Excipients |
|---|
|
Each film-coated tablet of EZETAST contains the following inactive ingredients: Povidone, sodium lauryl sulfate, lactose monohydrate, crospovidone, colloidal anhydrous silica, sodium stearylfumarate, microcrystalline cellulose, lactose, calcium carbonate, hyprolose and Opadry AMB complete film coating system OY-B-28920 White (ARTG No 4271). The film coating contains: Polyvinyl Alcohol, Titanium Dioxide, Talc, Lecithin and Xanthan Gum. |
Pack sizes and marketing
EZETAST tablets are packed in blisters of Cold form Alu-Alu as forming (base) material and 0.025 hard tampered heat-sealed lacquer coated plain Aluminium foil as the lidding material.
Available as 30 tablet blister (aluminium/aluminium) packs.
Marketing authorization holder
Lupin Australia Pty Ltd, Suite 2, Level 2, 19-23 Prospect Street, Box Hill, VIC, 3128
Distributor: Arrotex Pharmaceuticals Pty Ltd, 15-17 Chapel Street, Cremorne, VIC, 3121, Australia
Marketing authorization dates and numbers
Australian Registration Numbers:
EZETAST 10/10: AUST R 306963
EZETAST 10/20: AUST R 306966
EZETAST 10/40: AUST R 306958
EZETAST 10/80: AUST R 306956
Date of first approval: 01 November 2019
Drugs
| Drug | Countries | |
|---|---|---|
| EZETAST | Australia |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.