FENTORA Tablet Ref.[10810] Active ingredients: Fentanyl
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
FENTORA (fentanyl buccal tablet) is an opioid agonist, intended for buccal mucosal administration.
FENTORA is designed to be placed and retained within the buccal cavity for a period sufficient to allow disintegration of the tablet and absorption of fentanyl across the oral mucosa.
FENTORA employs the OraVescent drug delivery technology, which generates a reaction that releases carbon dioxide when the tablet comes in contact with saliva. It is believed that transient pH changes accompanying the reaction may optimize dissolution (at a lower pH) and membrane permeation (at a higher pH) of fentanyl through the buccal mucosa.
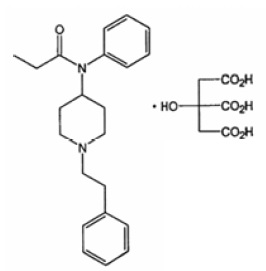
Active Ingredient: Fentanyl citrate, USP is N-(1-Phenethyl-4-piperidyl) propionanilide citrate (1:1).
Fentanyl is a highly lipophilic compound (octanol-water partition coefficient at pH 7.4 is 816:1) that is freely soluble in organic solvents and sparingly soluble in water (1:40). The molecular weight of the free base is 336.5 (the citrate salt is 528.6). The pKa of the tertiary nitrogens are 7.3 and 8.4.
The compound has the following structural formula:
All tablet strengths are expressed as the amount of fentanyl free base, e.g., the 100 microgram strength tablet contains 100 micrograms of fentanyl free base.
Inactive Ingredients: Mannitol, sodium starch glycolate, sodium bicarbonate, sodium carbonate, citric acid, and magnesium stearate.
| Dosage Forms and Strengths |
|---|
|
FENTORA tablets are flat-faced, round, beveled-edge in shape; are white in color; and are available in 100 mcg, 200 mcg, 400 mcg, 600 mcg, and 800 mcg strengths as fentanyl base. Each tablet strength is marked with a unique identifier [see How Supplied/Storage and Handling (16)]. |
| How Supplied | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
FENTORA is supplied in individually sealed, child-resistant blister packages. Each carton contains 7 blister cards with 4 white tablets in each card. The blisters are child-resistant, encased in peelable foil, and provide protection from moisture. Each tablet is debossed on one side with
Note: Carton/blister package colors are a secondary aid in product identification. Please be sure to confirm the printed dosage before dispensing. Distributed By: Teva Pharmaceuticals USA, Inc., North Wales, PA 19454 |
Drugs
| Drug | Countries | |
|---|---|---|
| FENTORA | Australia, Brazil, Canada, Hong Kong, Israel, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.
 , and the other side of each dosage strength is uniquely identified by the debossing on the tablet as described in the table below. In addition, the dosage strength is indicated on the blister package and the carton. See blister package and carton for product information.
, and the other side of each dosage strength is uniquely identified by the debossing on the tablet as described in the table below. In addition, the dosage strength is indicated on the blister package and the carton. See blister package and carton for product information.