FENTORA Tablet Ref.[10810] Active ingredients: Fentanyl
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Fentanyl is an opioid agonist whose principal therapeutic action is analgesia.
12.2. Pharmacodynamics
Effects on the Central Nervous System
The precise mechanism of the analgesic action is unknown although fentanyl is known to be a mu opioid receptor agonist. Specific CNS opioid receptors for endogenous compounds with opioid-like activity have been identified throughout the brain and spinal cord and play a role in the analgesic effects of this drug. Fentanyl produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem to both increases in carbon dioxide and to electrical stimulation.
Fentanyl causes miosis even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Fentanyl causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and in the duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone may be increased to the point of spasm resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of the sphincter of Oddi, and transient elevations in serum amylase.
Effects on the Cardiovascular System
Fentanyl produces peripheral vasodilation which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes and sweating, and/or orthostatic hypotension.
Effects on the Endocrine System
Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon [see Adverse Reactions (6.2]). Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6.2)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.
Concentration-Efficacy Relationships
The analgesic effects of fentanyl are related to the blood level of the drug, if proper allowance is made for the delay into and out of the CNS (a process with a 3- to 5-minute half-life).
In general, the effective concentration and the concentration at which toxicity occurs increase with increasing tolerance with any and all opioids. The rate of development of tolerance varies widely among individuals [see Dosage and Administration (2.1)].
The minimum effective analgesic concentration of fentanyl for any individual patient may increase over time due to an increase in pain, the development of a new pain syndrome and/or the development of analgesic tolerance [see Dosage and Administration (2.1, 2.4)].
Concentration-Adverse Reaction Relationships
There is a relationship between increasing fentanyl plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.1, 2.2, 2.3, 2.4)].
Respiratory System
All opioid mu-receptor agonists, including fentanyl, produce dose-dependent respiratory depression. The risk of respiratory depression is less in patients receiving chronic opioid therapy who develop tolerance to respiratory depression and other opioid effects. Peak respiratory depressive effects may be seen as early as 15 to 30 minutes from the start of oral transmucosal fentanyl citrate product administration and may persist for several hours.
Serious or fatal respiratory depression can occur even at recommended doses. Although not observed with oral transmucosal fentanyl products in clinical trials, fentanyl given rapidly by intravenous injection in large doses may interfere with respiration by causing rigidity in the muscles of respiration [see Warnings and Precautions (5.1)].
12.3. Pharmacokinetics
Fentanyl exhibits linear pharmacokinetics. Systemic exposure to fentanyl following administration of FENTORA increases linearly in an approximate dose-proportional manner over the 100- to 800-mcg dose range.
Absorption
Following buccal administration of FENTORA, fentanyl is readily absorbed with an absolute bioavailability of 65%. The absorption profile of FENTORA is largely the result of an initial absorption from the buccal mucosa, with peak plasma concentrations following venous sampling generally attained within an hour after buccal administration. Approximately 50% of the total dose administered is absorbed transmucosally and becomes systemically available. The remaining half of the total dose is swallowed and undergoes more prolonged absorption from the gastrointestinal tract.
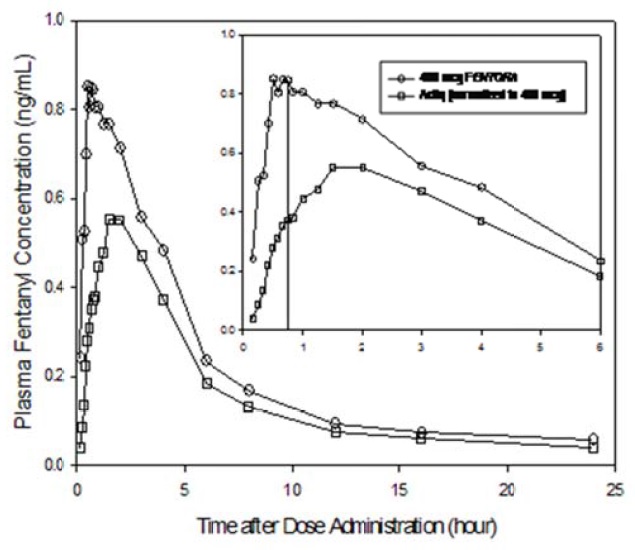
In a study that compared the absolute and relative bioavailability of FENTORA and ACTIQ (oral transmucosal fentanyl citrate), the rate and extent of fentanyl absorption were considerably different (approximately 30% greater exposure with FENTORA) (Table 5).
Table 5. Pharmacokinetic Parameters* in Adult Subjects Receiving FENTORA or ACTIQ:
| Pharmacokinetic Parameter (mean) | FENTORA 400 mcg | ACTIQ 400 mcg (adjusted dose)† |
|---|---|---|
| Absolute Bioavailability | 65% ± 20% | 47% ± 10.5% |
| Fraction Absorbed Transmucosally | 48% ± 31.8% | 22% ± 17.3% |
| Tmax (minute)‡ | 46.8 (20-240) | 90.8 (35-240) |
| Cmax (ng/mL) | 1.02 ± 0.42 | 0.63 ± 0.21 |
| AUC0-tmax (ng•hr/mL) | 0.40 ± 0.18 | 0.14 ± 0.05 |
| AUC0-inf (ng•hr/mL) | 6.48 ± 2.98 | 4.79 ± 1.96 |
* Based on venous blood samples.
† ACTIQ data was dose adjusted (800 mcg to 4 00 mcg).
‡ Data for Tmax presented as median (range).
Similarly, in another bioavailability study exposure following administration of FENTORA was also greater (approximately 50%) compared to Actiq.
Due to differences in drug delivery, measures of exposure (Cmax, AUC0-tmax, AUC0-inf) associated with a given dose of fentanyl were substantially greater with FENTORA compared to ACTIQ (see Figure 1). Therefore, caution must be exercised when switching patients from one product to another [see Dosage and Administration (2.2) and Warnings and Precautions (5.5)]. Figure 1 includes an inset which shows the mean plasma concentration versus time profile to 6 hours. The vertical line denotes the median Tmax for FENTORA.
Figure 1. Mean Plasma Concentration Versus Time Profiles Following Single Doses of FENTORA and ACTIQ in Healthy Subjects:
ACTIQ data was dose adjusted (800 mcg to 400 mcg).
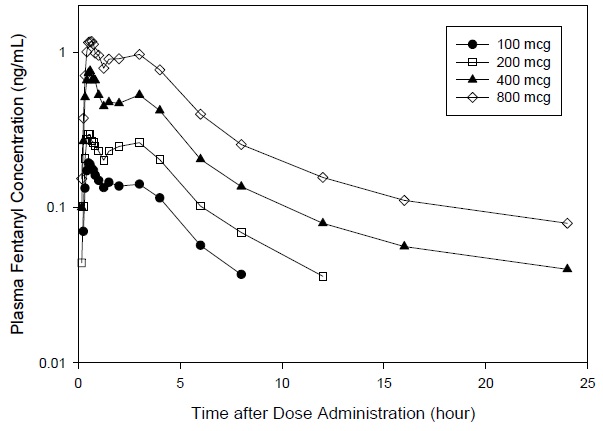
Mean pharmacokinetic parameters are presented in Table 6. Mean plasma concentration versus time profiles are presented in Figure 2.
Table 6. Pharmacokinetic Parameters?footnote? Following Single 100, 200, 400, and 800 mcg Doses of FENTORA in Healthy Subjects:
| Pharmacokinetic Parameter (mean±SD) | 100 mcg | 200 mcg | 400 mcg | 800 mcg |
|---|---|---|---|---|
| Cmax (ng/mL) | 0.25 ± 0.14 | 0.40 ± 0.18 | 0.97 ± 0.53 | 1.59 ± 0.90 |
| Tmax,minute† (range) | 45.0 (25.0 - 181.0) | 40.0 (20.0 - 180.0) | 35.0 (20.0 - 180.0) | 40.0 (25.0 - 180.0) |
| AUC0-inf (ng•hr/mL) | 0.98 ± 0.37 | 2.11 ± 1.13 | 4.72 ± 1.95 | 9.05 ± 3.72 |
| AUC0-tmax (ng•hr/mL) | 0.09 ± 0.06 | 0.13 ± 0.09 | 0.34 ± 0.23 | 0.52 ± 0.38 |
| T1/2, hr† | 2.63 (1.47 - 13.57) | 4.43 (1.85 - 20.76) | 11.09 (4.63 - 20.59) | 11.70 (4.63 - 28.63) |
* Based on venous sampling.
† Data for Tmax presented as median (range).
Figure 2. Mean Plasma Concentration Versus Time Profiles Following Single 100, 200, 400, and 800 mcg Doses of FENTORA in Healthy Subjects:
Dwell time (defined as the length of time that the tablet takes to fully disintegrate following buccal administration), does not appear to affect early systemic exposure to fentanyl.
The effect of mucositis (Grade 1) on the pharmacokinetic profile of FENTORA was studied in a group of patients with (N=8) and without mucositis (N=8) who were otherwise matched. A single 200 mcg tablet was administered, followed by sampling at appropriate intervals. Mean summary statistics (standard deviation in parentheses, expected tmax where range was used) are presented in Table 7.
Table 7. Pharmacokinetic Parameters in Patients with Mucositis:
| Patient status | Cmax (ng/mL) | tmax (min) | AUC0-tmax (ng•hr/mL) | AUC0-8 (ng•hr/mL) |
|---|---|---|---|---|
| Mucositis | 1.25 ± 0.78 | 25.0 (15 - 45) | 0.21 ± 0.16 | 2.33 ± 0.93 |
| No mucositis | 1.24 ± 0.77 | 22.5 (10 - 121) | 0.25 ± 0.24 | 1.86 ± 0.86 |
Following sublingual tablet placement, systemic exposure (as measured by AUC and Cmax) of fentanyl is equivalent to systemic exposure following buccal tablet placement.
Distribution
Fentanyl is highly lipophilic. The plasma protein binding of fentanyl is 80-85%. The main binding protein is alpha-1-acid glycoprotein, but both albumin and lipoproteins contribute to some extent. The mean oral volume of distribution at steady state (Vss/F) was 25.4 L/kg.
Elimination
Metabolism
The metabolic pathways following buccal administration of FENTORA have not been characterized in clinical studies. The progressive decline of fentanyl plasma concentrations results from the uptake of fentanyl in the tissues and biotransformation in the liver. Fentanyl is metabolized in the liver and in the intestinal mucosa to norfentanyl by cytochrome P450 3A4 isoform. In animal studies, norfentanyl was not found to be pharmacologically active [see Drug Interactions (7)].
Excretion
Disposition of fentanyl following buccal administration of FENTORA has not been characterized in a mass balance study. Fentanyl is primarily (more than 90%) eliminated by biotransformation to N-dealkylated and hydroxylated inactive metabolites. Less than 7% of the administered dose is excreted unchanged in the urine, and only about 1% is excreted unchanged in the feces. The metabolites are mainly excreted in the urine, while fecal excretion is less important.
The total plasma clearance of fentanyl following intravenous administration is approximately 42 L/h.
Sex
Systemic exposure was higher for women than men (mean Cmax and AUC values were approximately 28% and 22% higher, respectively). The observed differences between men and women were largely attributable to differences in weight.
Race
In studies conducted in healthy Japanese subjects, systemic exposure was generally higher than that observed in U.S. subjects (mean Cmax and AUC values were approximately 50% and 20% higher, respectively). The observed differences were largely attributed to the lower mean weight of the Japanese subjects compared to U.S. subjects (57.4 kg versus 73 kg).
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Fentanyl was evaluated for carcinogenic potential in a 104-week rat study and in a 6-month Tg.AC transgenic mouse study. In rats, doses up to 50 mcg/kg in males and 100 mcg/kg in females were administered subcutaneously and no treatment-related neoplasms were observed (doses are equivalent to 2.3- and 3.4-times the exposure of a single human dose of 800 mcg per pain episode, respectively, based on an AUC comparison). In a 26-week transgenic mice model (Tg.AC), at topical doses up to 50 mcg/dose/day, no increase in the occurrence of treatment-related neoplasms was observed.
Mutagenesis
Fentanyl citrate was not mutagenic in the Ames reverse mutation assay in S. typhimurium or E. coli, or the mouse lymphoma mutagenesis assay. Fentanyl citrate was not clastogenic in the in vivo mouse micronucleus assay.
Impairment of Fertility
In a fertility study, female rats were administered fentanyl subcutaneously for 14 days prior to mating with untreated males at doses up to 300 mcg/kg and no effects on female fertility were observed. The systemic exposure at the dose of 300 mcg/kg was approximately 8.6 times the exposure of a single human dose of 800 mcg per pain episode, based on an AUC comparison. Males were administered fentanyl subcutaneously for 28 days prior to mating with untreated females at doses up to 300 mcg/kg. At 300 mcg/kg, adverse effects on sperm parameters, which affected fertility, were observed. These effects included decreased percent mobile sperm, decreased sperm concentrations as well as an increase in the percent abnormal sperm. The dose in males at which no effects on fertility were observed was 100 mcg/kg, which is approximately 5.7- times the exposure of a single human dose of 800 mcg per pain episode, based on an AUC comparison.
Fentanyl has been shown to impair fertility in rats at doses of 30 mcg/kg IV and 160 mcg/kg subcutaneously. Conversion to the human equivalent doses indicates that this is within the range of the human recommended dosing for FENTORA.
14. Clinical Studies
The efficacy of FENTORA was demonstrated in a double-blind, placebo-controlled, cross-over study in opioid tolerant patients with cancer and breakthrough pain. Patients considered opioid tolerant were those who were taking at least 60 mg of oral morphine daily, at least 25 mcg/hour of transdermal fentanyl, at least 30 mg of oral oxycodone daily, at least 8 mg of oral hydromorphone daily or an equianalgesic dose of another opioid daily for a week or longer.
In this trial, patients were titrated in an open-label manner to a successful dose of FENTORA. A successful dose was defined as the dose in which a patient obtained adequate analgesia with tolerable side effects. Patients who identified a successful dose were randomized to a sequence of 10 treatments with 7 being the successful dose of FENTORA and 3 being placebo. Patients used one tablet of study drug (either FENTORA or placebo) per breakthrough pain episode.
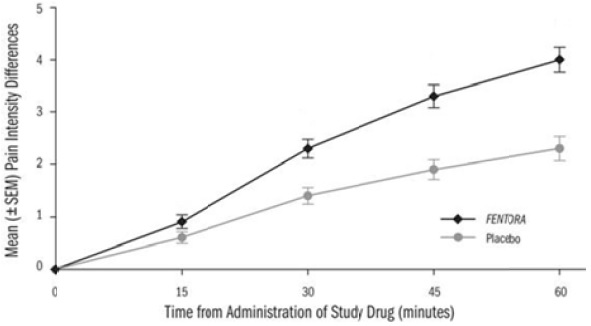
Patients assessed pain intensity on a scale that rated the pain as 0=none to 10=worst possible pain. With each episode of breakthrough pain, pain intensity was assessed first and then treatment was administered. Pain intensity (0-10) was then measured at 15, 30, 45, and 60 minutes after the start of administration. The sum of differences in pain intensity scores at 15 and 30 minutes from baseline (SPID30) was the primary efficacy measure.
Sixty-five percent (65%) of patients who entered the study achieved a successful dose during the titration phase. The distribution of successful doses is shown in Table 8. The median dose was 400 mcg.
Table 8. Successful Dose of FENTORA Following Initial Titration:
| FENTORA Dose | n (%) (N=80) |
|---|---|
| 100 mcg | 13 (16) |
| 200 mcg | 11 (14) |
| 400 mcg | 21 (26) |
| 600 mcg | 10 (13) |
| 800 mcg | 25 (31) |
The LS mean (SE) SPID30 for FENTORA-treated episodes was 3.0 (0.12) while for placebo-treated episodes it was 1.8 (0.18).
Figure 3. Mean Pain Intensity Differences (PID) at Each Time Point During the Double-Blind Treatment Period
PID=pain intensity difference; SEM=standard error of the mean
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.