GIAPREZA Solution for injection Ref.[9974] Active ingredients: Angiotensin II
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
Angiotensin II is a naturally occurring peptide hormone of the renin-angiotensin-aldosterone system (RAAS) that causes vasoconstriction and an increase in blood pressure. GIAPREZA is a sterile, aqueous solution of synthetic human angiotensin II for intravenous administration by infusion. Each 1 mL of GIAPREZA contains 2.5 mg angiotensin II equivalent to an average of 2.9 mg angiotensin II acetate, 25 mg mannitol, and Water for Injection adjusted with sodium hydroxide and/or hydrochloric acid to pH of 5.5.
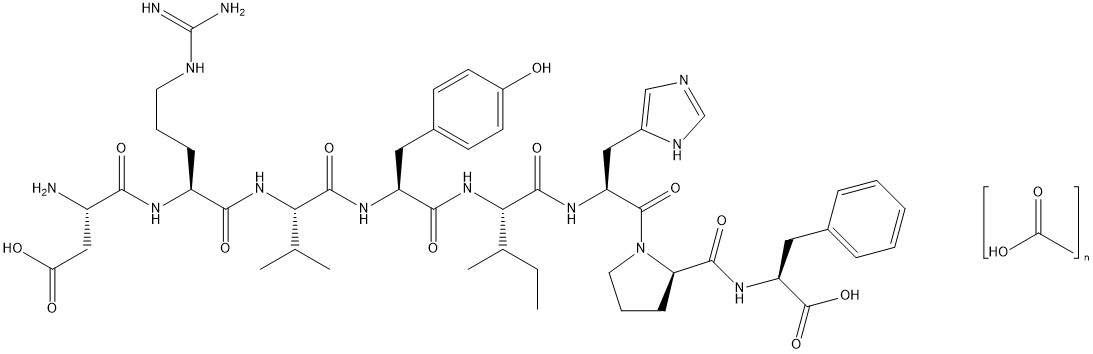
The chemical name of the synthetic angiotensin II acetate is L-Aspartyl-L-arginyl-L-valyl-Ltyrosyl-L-isoleucyl-L-histidyl-L-prolyl-L-phenylalanine, acetate salt. The counter ion acetate is present in a non-stoichiometric ratio. It is a white to off-white powder, soluble in water.
The structure of angiotensin II acetate is shown below.
Molecular formula: C50H71N13O12∙ (C2H4O2)n; (n = number of acetate molecules; theoretical n = 3)
Average molecular weight: 1046.2 (as free base).
| Dosage Forms and Strengths |
|---|
|
Injection: 2.5 mg/mL angiotensin II in a vial. GIAPREZA is a clear, aqueous solution. |
| How Supplied |
|---|
|
GIAPREZA (angiotensin II) Injection is a clear, aqueous solution for administration by intravenous infusion supplied as a single-dose vial:
Manufactured for: La Jolla Pharmaceutical Company, San Diego, CA 92121 |
Drugs
| Drug | Countries | |
|---|---|---|
| GIAPREZA | Austria, Estonia, Finland, France, Croatia, Ireland, Italy, Lithuania, Poland, Romania, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.