GIAPREZA Solution for injection Ref.[9974] Active ingredients: Angiotensin II
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Angiotensin II raises blood pressure by vasoconstriction and increased aldosterone release. Direct action of angiotensin II on the vessel wall is mediated by binding to the G-protein-coupled angiotensin II receptor type 1 on vascular smooth muscle cells, which stimulates Ca 2+/calmodulin-dependent phosphorylation of myosin and causes smooth muscle contraction.
12.2. Pharmacodynamics
For the 114 (70%) patients in the GIAPREZA arm who reached the target MAP at Hour 3, the median time to reach the target MAP endpoint was approximately 5 minutes. GIAPREZA is titrated to effect for each individual patient.
12.3. Pharmacokinetics
Following intravenous infusion of angiotensin II in adults with septic or other distributive shock, serum levels of angiotensin II are similar at Baseline and Hour 3 after intravenous infusion. After 3 hours of treatment, however, the serum level of angiotensin I (the angiotensin II precursor peptide) is reduced by approximately 40%.
Distribution
No specific studies were conducted that examined the distribution of GIAPREZA.
Metabolism and Excretion
No specific studies were conducted that examined the metabolism and excretion of GIAPREZA.
The plasma half-life of IV administered angiotensin II is less than one minute. It is metabolized by aminopeptidase A and angiotensin converting enzyme 2 to angiotensin-(2-8) [angiotensin III] and angiotensin-(1-7), respectively in plasma, erythrocytes and many of the major organs (i.e., intestine, kidney, liver and lung). Angiotensin II type 1 receptor (AT1) mediated activity of angiotensin III is approximately 40% of angiotensin II; however, aldosterone synthesis activity is similar to angiotensin II. Angiotensin-(1-7) exerts the opposite effects of angiotensin II on AT1 receptors and causes vasodilation.
Specific Populations
No formal pharmacokinetic studies were conducted with GIAPREZA in the following specific populations.
Renal Impairment
The clearance of angiotensin II is not dependent on renal function. Therefore, the pharmacokinetics of GIAPREZA are not expected to be influenced by renal impairment.
Hepatic Impairment
The clearance of angiotensin II is not dependent on hepatic function. Therefore, the pharmacokinetics of GIAPREZA are not expected to be influenced by hepatic impairment.
Age
The effect of age was analyzed in the 163 patients receiving GIAPREZA in ATHOS-3. There were no significant differences in pharmacokinetics between age groups (<65 years / ≥65 years).
Male and Female Patients
The effect of sex was analyzed in the 163 patients receiving GIAPREZA in ATHOS-3. There were no significant differences in pharmacokinetics between male and female patients.
13. Nonclinical Toxicology
13.3. Safety Pharmacology
In a cardiovascular safety pharmacology study in normotensive dogs, GIAPREZA doses of 150, 450, and 1,800 ng/kg (5, 15, and 60 ng/kg/min) were infused intravenously for 30 minutes each. At ≥450 ng/kg, GIAPREZA caused significantly elevated MAP and systemic vascular resistance, as expected. The 1,800 ng/kg dose also caused increased heart rate, increased systemic vascular resistance, increased left ventricular systolic and end-diastolic pressures, and PR interval prolongation. GIAPREZA did not significantly alter respiratory rate or cause electrocardiographic changes in QRS duration or QTc.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No genetic toxicity studies have been conducted with GIAPREZA. No carcinogenicity or fertility studies with GIAPREZA have been conducted in animals.
13.2. Animal Toxicology and/or Pharmacology
No animal toxicology studies were conducted with GIAPREZA.
14. Clinical Studies
14.1. ATHOS-3
The Angiotensin II for the Treatment of High-Output Shock (ATHOS-3) trial was a double-blind study in which 321 adults with septic or other distributive shock who remained hypotensive despite fluid and vasopressor therapy were randomized 1:1 and treated with either GIAPREZA or placebo, both in addition to background vasopressor therapy. Doses of GIAPREZA or placebo were titrated to a target MAP of ≥75 mmHg during the first 3 hours of treatment while doses of other vasopressors were maintained. From Hour 3 to Hour 48, GIAPREZA or placebo were titrated to maintain MAP between 65 and 70 mmHg while reducing doses of other vasopressors. The primary endpoint was the percentage of subjects who achieved either a MAP ≥75 mmHg or a ≥10 mmHg increase in MAP without an increase in baseline vasopressor therapy at 3 hours.
91% of subjects had septic shock; the remaining subjects had other forms of distributive shock such as neurogenic shock. At the time of study drug administration, 97% of subjects were receiving norepinephrine, 67% vasopressin, 15% phenylephrine, 13% epinephrine, and 2% dopamine. 83% of subjects had received two or more vasopressors and 47% three or more vasopressors prior to study drug administration. 61% of subjects were male, 80% were White, 10% were Black, and 10% were other races. The median age of subjects was 64 years (range: 22-89 years). Patients requiring high doses of steroids, patients with a history of asthma or bronchospasm, and patients with Raynaud’s syndrome were not included.
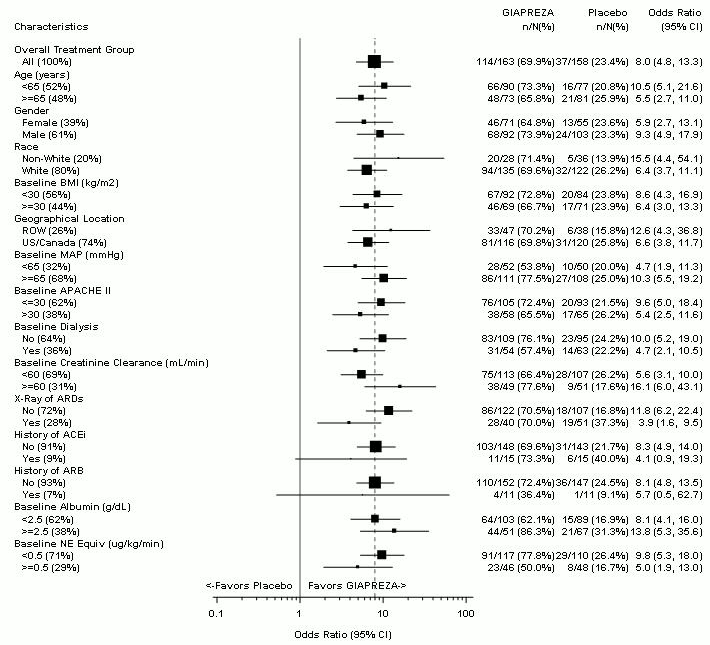
The primary endpoint was achieved by 70% of patients randomized to GIAPREZA compared to 23% of placebo subjects; p <0.0001 (a treatment effect of 47%). Figure 1 shows the results in all patients and in selected subgroups.
Figure 1: ATHOS-3: Primary Endpoint – Overall Result and Results in Selected Subgroups
NE Equiv = norepinephrine equivalent dose: the sum of all vasopressors doses with each vasopressor dose converted to the clinically equivalent norepinephrine dose
Note: The figure above presents effects in various subgroups, all of which are baseline characteristics. The 95% confidence limits that are shown do not take into account the number of comparisons made and may not reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
In the GIAPREZA-treated group, the median time to reach the target MAP endpoint was 5 minutes. The effect on MAP was sustained for at least the first three hours of treatment. The median dose of GIAPREZA was 10 ng/kg/min at 30 minutes. Of the 114 responders at Hour 3, only 2 (1.8%) received more than 80 ng/kg/min.
Patients were not necessarily on maximum doses of other vasopressors at the time of randomization. The effect of GIAPREZA when added to maximum doses of other vasopressors is unknown.
Mortality through Day 28 was 46% on GIAPREZA and 54% on placebo (hazard ratio 0.78; 95% confidence interval 0.57-1.07).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.