LUTRATE DEPOT Powder and solvent for prolonged-release suspension for injection Ref.[6974] Active ingredients: Leuprorelin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2017 Publisher: Mercury Pharmaceuticals Limited, Capital House, 85 King William Street, London EC4N 7BL, United Kingdom
Therapeutic indications
- Metastatic prostate cancer.
- Locally advanced prostate cancer, as an alternative to surgical castration.
- As an adjuvant treatment to radiotherapy in patients with high-risk localised or locally advanced prostate cancer.
- As an adjuvant treatment to radical prostatectomy in patients with locally advanced prostate cancer at high risk of disease progression.
- As neo-adjuvant treatment prior to radiotherapy in patients with high-risk localised or locally advanced prostate cancer.
Posology and method of administration
Posology
The usual recommended dose of Lutrate 1 month Depot is 3.75 mg presented as a one month depot injection and administered as a single intramuscular injection every month.
Lutrate 1 month Depot must be administered under the supervision of a physician or a qualified health practitioner.
The dose of Lutrate 1 month Depot allowing the continuous release of leuprorelin acetate during one month is incorporated in a depot formulation. The lyophilized powder should be reconstituted and administered as a single intramuscular injection at monthly intervals. Intra-arterial or intravenous administration must be avoided. The vial of
Lutrate 1 month Depot microsphere powder should be reconstituted immediately prior to administration by intramuscular injection. As with other drugs administered regularly by injection, the injection site should be varied periodically. Lutrate 1 month Depot therapy should not be discontinued when remission or improvement occurs.
Response to Lutrate 1 month Depot therapy should be monitored measuring serum levels of testosterone as well as prostate-specific antigen (PSA) periodically. Clinical studies have shown that testosterone levels increased during the first 4 days of treatment in the majority of non-orchiectomised patients. They then decreased and reached castrate levels by 3-4 weeks. Once attained, castrate levels (defined as concentration of testosterone less than 0.5 ng/mL) were maintained as long as drug therapy continued.
If a patient's response appears to be sub-optimal, then it would be advisable to confirm that serum testosterone levels have reached or are remaining at castrate levels. Transient increases in acid phosphatase levels sometimes occur early in the treatment period but usually return to normal or near normal values by the 4th week of treatment.
Duration of treatment
Lutrate 1 month Depot should be administered as monthly intramuscular injections. As a rule, prostate cancer therapy with Lutrate 1 month Depot entails long-term treatment and therapy should not be discontinued when remission or improvement occurs.
Special populations
Paediatric population
The safety and efficacy of Lutrate 1 month Depot in the paediatric patients has not been established. Therefore, Lutrate 1 month Depot is not recommended in children or adolescents until safety and efficacy data become available.
Renal/hepatic impairment
The pharmacokinetics of Lutrate 1 month Depot in hepatically and renally impaired patients has not been determined.
Elderly
In the clinical trial for Lutrate 1 month Depot, the mean age of the subjects studied was 71.6 ± 9.2 years. Therefore, the labelling reflects the pharmacokinetics, efficacy and safety of Lutrate 1 month Depot in this population.
Method of administration
Lutrate 1 month Depot must be administered via the intramuscular route only. Do not administer by any other route. If it is administered subcutaneously by mistake, the patient should be closely monitored since no data about other administration routes apart from intramuscular is available for Lutrate 1 month Depot. For instructions on reconstitution of the medicinal product before administration, see section 6.6.
Overdose
There is no clinical experience with the effects of an acute overdose of Lutrate 1 month Depot or leuprorelin acetate. In clinical trials using daily subcutaneous leuprorelin acetate in patients with prostate cancer, doses as high as 20 mg/day for up to two years caused no AEs differing from those observed with the 1 mg/day dose.
In animal studies, doses of up to 500 times the recommended human dose resulted in dyspnoea, decreased activity and local irritation at the injection site. In cases of overdosage, the patient should be monitored closely and management should be symptomatic and supportive.
Shelf life
Shelf life: 3 years unopened.
Once reconstituted with the solvent the suspension should be administered immediately.
Special precautions for storage
Do not store above 25°C. Do not freeze.
Store in the original package in order to protect from light.
For storage conditions after reconstitution of the medicinal product, see section 6.3.
Nature and contents of container
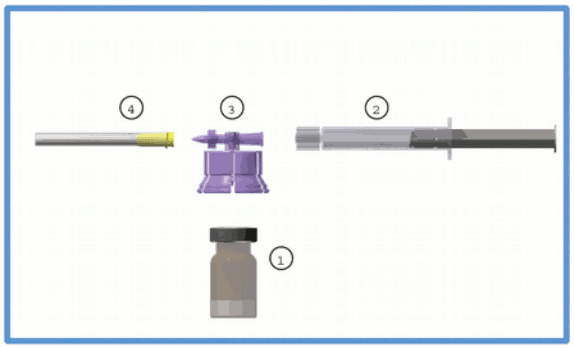
The commercial kit includes:
- One (1) type I glass vial containing 3.75 mg of leuprorelin acetate as a freeze-dried powder, sealed with a bromobutyl stopper and an aluminium flip-off cap.
- One (1) type I glass prefilled syringe containing 2 ml of solvent sealed with an elastomer cap.
- One (1) polycarbonate / HDPE adaptor system including one (1) sterile 20 gauge needle.
Special precautions for disposal and other handling
Method of administration
The vial of Lutrate 1 month Depot microsphere powder should be reconstituted immediately prior to administration by intramuscular injection. Make sure an aseptic technique is followed.
The reconstituted product is a suspension of milky, white colour appearance.
No other solvent can be used for reconstitution of Lutrate 1 month Depot.
Reconstitute Lutrate 1 month Depot according to the following instructions:
Some product may cake or clump at the vial wall. This is considered normal. During product manufacture the vial is filled with excess product in order to make sure that a final dose of 3.75 mg of leuprorelin acetate is administered.
The product is meant for a single injection. Any remaining suspension must be discarded.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.