METAGYL Tablet Ref.[50556] Active ingredients: Methronidazole
Source: Marketing Authorisation Holder Revision Year: 2022 Publisher: Oethmaan Biosims (PTY) Ltd., 207A Sherwood House, Greenacres Office Park, c/o Victory and Rustenburg Roads, Victory Park, Johannesburg 2195
4.1. Therapeutic indications
a) In the oral treatment of:
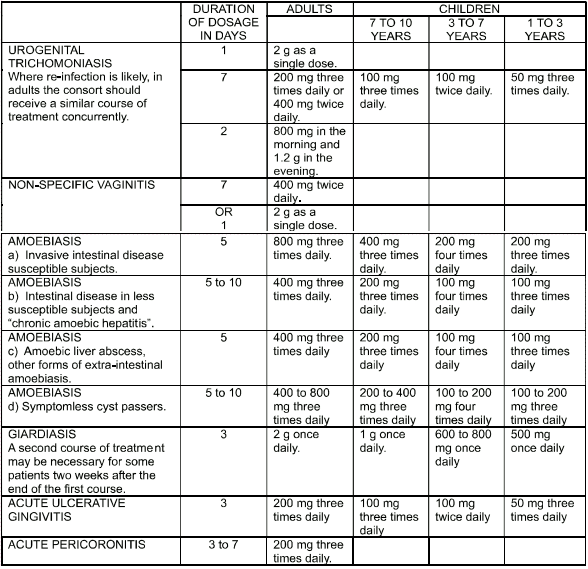
- urogenital trichomoniasis
- nonspecific vaginitis
- all forms of amoebiasis
- giardiasis
- acute ulcerative gingivitis (Vincent’s)
- acute pericoronitis
b) Treatment of infections in which anaerobic bacteria have been identified or are suspected as pathogens, particularly Bacteroides fragilis and other species of bacteroides and including other species for which metronidazole is bactericidal, such as fusobacteria, clostridia, eubacteria and anaerobic streptococci.
Metronidazole has been used successfully for anaerobic infections in the following conditions: pelvic inflammatory disease and postoperative wound infections. Combined therapy is often indicated as there are usually mixed infections.
c) Prevention of postoperative infections due to anaerobic bacteria:
i. Given before and after gynaecological surgery.
ii. Given before and after appendicectomy.
iii. Given before and after colonic surgery.
d) Treatment of Helicobacter pylori-associated gastritis and duodenal ulcer. Metronidazole is used in combination with bismuth subsalicylate or colloidal bismuth subcitrate and appropriate antibiotic therapy.
4.2. Posology and method of administration
The tablets should betaken with or afterfood. Immature children and babies weighing less than 10 kg should receive proportionally smalle doses, as advised by the physician. Children over 10 years may be given a suitable proportion o he adult dosage according to bodymass.
Anaerobic Infection
a) Treatment
METAGYL tablets may be given alone or concurrently with other bacteriologically appropriate antibacterial agents. They should be given for 7 days or longer depending on clinical and bacteriological assessments of the patient’s condition.
Adults: Initially, 800 mg followed by 400 mg by mouth every 8 hours.
Children: 7,5 mg/kg bodymass by mouth every 8 hours.
b) Prevention
Adults: Administered in doses similar to those used for the treatment of established infection. 400 mg may be given every 8 hours in the 24 hours before surgery followed postoperatively by intravenous or rectal administration until oral therapy is possible.
Children: as for treatment (a)
Treatment of Helicobacter pylori-associated gastritis and duodenal ulcer.
| Heliobacter pylori-associated gastritis or duodenal ulcer (treatment adjunct) | -500 mg three times a day in conjunction with other medicines used for treatment (ie., bismuth subsalicylate, colloidal bismuth subcitrate, ampicillin or amoxicillin) for one to two weeks. |
4.9. Overdose
See side effects: Treatment is symptomatic and supportive.
6.4. Special precautions for storage
Keep in well closed in light-resistant containers, at or below 25°C.
KEEP OUT OF REACH OF CHILDREN.
6.5. Nature and contents of container
Metagyl Tablets: Blisters and patient ready packs of 21’s, 28’s or 250’s.
HDPE Bottle of 1000’s.
Metagyl Forte Tablets: Blisters and patient ready packs of 5’5, 14’s, 21’s or 100’s.
HDPE Bottle of 500’s.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.