MODURETIC Tablet Ref.[50873] Active ingredients: Amiloride Hydrochlorothiazide
Source: Medicines and Medical Devices Safety Authority (NZ) Revision Year: 2021 Publisher: Pharmacy Retailing Pty Ltd, t/a Healthcare Logistics, 58 Richard Pearse Drive, Airport Oaks, Auckland, New Zealand Telephone (09) 9185 100 Email: aspen@aspenpharma.co.nz
5.1. Pharmacodynamic properties
MODURETIC (amiloride HCl and hydrochlorothiazide) combines the potassium-conserving action of amiloride HCl with the natriuretic action of hydrochlorothiazide.
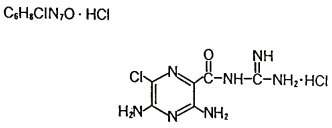
Amiloride HCl is a pyrazinecarbonylguanidine that is unrelated chemically to other known diuretic or antikaliuretic agents. It is not acidic like the thiazides or ethacrynic acid, but is the salt of a moderately strong base, amiloride, pKa 8.7.
Its chemical name is 3,5-diamino-N-(aminoimineomethyl)-6-chloropyrazinecarboxamide monohydrochloride and its structure is as follows:
The CAS Number is: 17440-83-4.
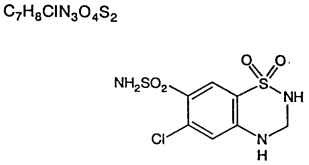
Hydrochlorothiazide is the 3,4-dihydro derivative of chlorothiazide (CHLOTRIDE*). It is a white, or practically white, crystalline compound, slightly soluble in water, but freely soluble in sodium hydroxide solution. Its chemical name is:6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulphonamide 1,1-dioxide, and its structure is as follows:
The CAS Number is: 58-93-5.
Mechanism of Action
MODURETIC provides diuretic and antihypertensive activity (principally due to the hydrochlorothiazide component), while acting through the amiloride component to prevent the excessive potassium loss that may occur in patients receiving a thiazide diuretic. Due to its amiloride component, the urinary excretion of magnesium is less with MODURETIC than with a thiazide or loop diuretic used alone (see PRECAUTIONS). The onset of the diuretic action of MODURETIC is within 1 to 2 hours and this action appears to be sustained for approximately 24 hours.
Amiloride HCl
Amiloride HCl is a potassium-conserving (antikaliuretic) drug that possesses weak (compared to the thiazide diuretics) natriuretic, diuretic and antihypertensive activity. These effects have been partially additive to the effects of thiazide diuretics in some clinical studies. Amiloride HCl has potassium-conserving activity in patients receiving kaliuretic-diuretic agents.
Amiloride HCl is not an aldosterone antagonist and the effects are seen even in the absence of aldosterone.
Amiloride HCl exerts its potassium sparing effect through the inhibition of sodium reabsorption at the distal convoluted tubule, cortical collecting tubule and collecting duct; this decreases the net negative potential of the tubular lumen and reduces both potassium and hydrogen secretion and their subsequent excretion. This mechanism accounts in large part for the potassium sparing action of amiloride.
Hydrochlorothiazide
The mechanism of the antihypertensive effect of thiazides is unknown. Hydrochlorothiazide does not usually affect normal blood pressure. Hydrochlorothiazide is a diuretic and antihypertensive agent. It affects the renal tubular mechanism of electrolyte reabsorption. Hydrochlorothiazide increases excretion of sodium and chloride in approximately equivalent amounts. Natriuresis may be accompanied by some loss of potassium and bicarbonate. After oral use diuresis begins within two hours, peaks in about four hours and lasts about 6 to 12 hours.
Non-melanoma skin cancer: Based on available data from epidemiological studies, cumulative dose-dependent association between HCTZ and NMSC has been observed. One study included a population comprised of 71,533 cases of BCC and of 8,629 cases of SCC matched to 1,430,833 and 172,462 population controls, respectively. High HCTZ use (≥50,000mg cumulative) was associated with an adjusted OR of 1.29 (95% CI: 1.23-1.35) for BCC and 3.98 (95%CI: 3.68-4.31) for SCC. A clear cumulative dose response relationship was observed for both BCC and SCC. Another study showed a possible association between lip cancer (SCC) and exposure to HCTZ: 633 cases of lip-cancer were matched with 63,067 population controls, using a risk-set sampling strategy. A cumulative dose-response relationship was demonstrated with an adjusted OR 2.1 (95% CI: 1.7-2.6) increasing to OR 3.9 (3.0-4.9) for high use (~25,000mg) and OR 7.7 (5.7-10.5) for the highest cumulative dose (100,000 mg) (see also section 4.4).
5.2. Pharmacokinetic properties
Amiloride HCl usually begins to act within two hours after an oral dose. Its effect on electrolyte excretion reaches a peak between 6 and 10 hours and lasts about 24 hours. Peak plasma levels are obtained in 3 to 4 hours and the plasma half-life varies from 6 to 9 hours. Effects on electrolytes increase with single doses of amiloride HCl up to approximately 15 mg.
Amiloride HCl is not metabolised by the liver but is excreted unchanged by the kidneys. About 50 percent of a 20 mg dose of amiloride HCl is excreted in the urine and 40 percent in the stool within 72 hours. Amiloride HCl has little effect on glomerular filtration rate or renal blood flow. Because amiloride HCl is not metabolised by the liver, drug accumulation is not anticipated in patients with hepatic dysfunction, but accumulation can occur if the hepatorenal syndrome develops.
Hydrochlorothiazide is not metabolised but is eliminated rapidly by the kidney. When plasma levels have been followed for a least 24 hours, the plasma half-life has been observed to vary between 5.6 and 14.8 hours. At least 61 percent of the oral dose is eliminated unchanged within 24 hours. Hydrochlorothiazide crosses the placenta but not the blood-brain barrier and is excreted in breast milk.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.