NORGESIC Tablet, uncoated Ref.[50936] Active ingredients: Orphenadrine Paracetamol
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2021 Publisher: iNova Pharmaceuticals (Australia) Pty Limited, Level 10, 12 Help Street, Chatswood NSW 2067, Australia, Tel: 1800 630 056
Product name and form
NORGESIC (Orphenadrine and paracetamol).
| Pharmaceutical Form |
|---|
|
Tablet, uncoated. White, scored, immediate release tablets marked N/C on one side and no markings on the other side. |
Qualitative and quantitative composition
Orphenadrine citrate 35 mg and paracetamol 450 mg tablets.
For the full list of excipients, see Section 6.1 List of excipients.
Physicochemical property
Orphenadrine citrate is white or almost white, crystalline powder. It is sparingly soluble in water, and slightly soluble in alcohol. Paracetamol is a white or almost white, crystalline powder that is sparingly soluble in water and freely soluble in alcohol.
Chemical structure
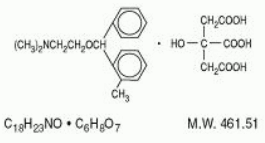
Orphenadrine citrate
Chemical name: (RS)-N,N-Dimethyl-2-[(2-methylphenyl)phenylmethoxy]ethanamine dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
Chemical structure:
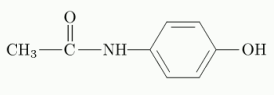
Paracetamol
Chemical name: N-(4-Hydroxyphenyl)acetamide
Chemical structure:
CAS number
Orphenadrine citrate: 4682-36-4
Paracetamol: 103-90-2
| Active Ingredient |
|---|
|
Orphenadrine, which is a congener of diphenhydramine without sharing its soporific effect, is an anti-muscarinic agent. Orphenadrine is used as the hydrochloride in the symptomatic treatment of Parkinsonism. |
|
Paracetamol is a medication used to treat pain and fever. It does appear to selectively inhibit COX activities in the brain, which may contribute to its ability to treat fever and pain. |
| List of Excipients |
|---|
|
Microcrystalline cellulose, magnesium stearate, colloidal anhydrous silica, gelatin and pregelatinised maize starch. |
Pack sizes and marketing
PP jars with PP child-resistant closure: 8 (sample pack), 24, 100, and 500s#
PVC/Al blisters: 8 (sample pack), 24, 100’s.
# - not marketed.
Marketing authorization holder
iNova Pharmaceuticals (Australia) Pty Limited, Level 10, 12 Help Street, Chatswood NSW 2067, Australia, Tel: 1800 630 056
Marketing authorization dates and numbers
4 July 1991
Drugs
| Drug | Countries | |
|---|---|---|
| NORGESIC | Austria, Finland, Hong Kong, Malta, New Zealand, Singapore |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.