NOYADA Oral solution Ref.[27955] Active ingredients: Captopril
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: Martindale Pharmaceuticals Limited (T/S Martindale Pharma), Bampton Road, Harold Hill, Essex, RM3 8UG
Product name and form
Noyada 5mg/5ml Oral Solution.
| Pharmaceutical Form |
|---|
|
Oral solution. Clear, colourless solution. |
Qualitative and quantitative composition
Each ml of 5mg/5ml oral solution contains 1mg captopril.
Excipient(s) with known effects: Each mL of Noyada oral solution contains 0.017 mmol (or 0.391 mg) sodium including 0.5 mg sodium benzoate (E211).
For the full list of excipients, see section 6.1.
| Active Ingredient |
|---|
|
Captopril is a highly specific, competitive inhibitor of angiotensin-I converting enzyme (ACE inhibitors). Inhibition of ACE results in decreased plasma angiotensin-II, which leads to decreased vasopressor activity and to reduced aldosterone secretion. In patients with hypertension, captopril causes a reduction in supine and erect blood pressure, without inducing any compensatory increase in heart rate, nor water and sodium retention. |
| List of Excipients |
|---|
|
Disodium EDTA |
Pack sizes and marketing
mber glass bottle with child resistant and tamper evident caps. Each bottle is packed in a cardboard carton containing 1ml and 5ml syringes with an adaptor along with a patient leaflet.
5 mg/5 ml oral solution.
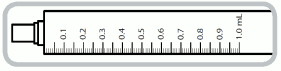
1 ml syringe- each numbered increment is 0.1 ml equivalent to 0.1 mg of captopril. The intermediate increments are 0.05 ml equivalent to 0.05 mg of captopril.
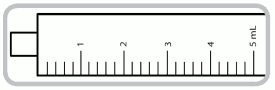
5 ml syringe- each numbered increment is 1 ml equivalent to 1 mg of captopril. The intermediate increments are 0.2 ml equivalent to 0.2 mg of captopril.
Pack size: 100ml
Marketing authorization holder
Martindale Pharmaceuticals Limited (T/S Martindale Pharma), Bampton Road, Harold Hill, Essex, RM3 8UG
Marketing authorization dates and numbers
PL 00156/0361
Date of first authorisation: 29/05/2013
Date of latest renewal: 24/04/2018
Drugs
| Drug | Countries | |
|---|---|---|
| NOYADA | France, United Kingdom |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.