NOYADA Oral solution Ref.[27955] Active ingredients: Captopril
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2021 Publisher: Martindale Pharmaceuticals Limited (T/S Martindale Pharma), Bampton Road, Harold Hill, Essex, RM3 8UG
4.1. Therapeutic indications
Hypertension: Noyada oral solution is indicated for the treatment of hypertension.
Heart Failure: Noyada oral solution is indicated for the treatment of chronic heart failure with reduction of systolic ventricular function, in combination with diuretics and, when appropriate, digitalis and beta-blockers.
Myocardial Infarction:
- short-term (4 weeks) treatment: Noyada oral solution is indicated in any clinically stable patient within the first 24 hours of an infarction.
- long-term prevention of symptomatic heart failure: Noyada oral solution is indicated in clinically stable patients with asymptomatic left ventricular dysfunction (ejection fraction equal to or below 40%).
Type I Diabetic Nephropathy: Noyada oral solution is indicated for the treatment of macroproteinuric diabetic nephropathy in patients with type I diabetes. (See section 5.1).
4.2. Posology and method of administration
Noyada oral solution is available in two strengths 5mg/5ml and 25mg/5ml;
For lower doses that include fractions of a mg, the 5mg/5ml product should be used.
For higher doses the 25mg/5ml product is recommended.
The following table provides a guide for using Noyada 5mg/5ml or Noyada 25mg/5ml for most common dose.
| Dose | Noyada 5mg/5ml | Noyada 25mg/5ml | |
|---|---|---|---|
| Adult population | 6.25 mg | 6.25ml | |
| 12.5 mg | 12.5 ml | ||
| 25 mg | 5ml | ||
| 37.5 mg | 7.5ml | ||
| 50mg | 10ml | ||
| 75mg | 15ml | ||
| 100mg | 20ml | ||
| 150mg | 30ml | ||
| Paediatric population | 0.15mg/kg | 0.15ml/kg | |
| 0.3mg/kg | 0.3ml/kg |
For further information on measuring the dose please see section 6.5
The 5mg/5ml product is supplied with the following administration devices:
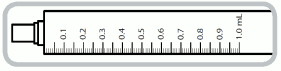
- 1 mL syringe graduated with numbered increments of 0.1mL (= 0.1 mg captopril) and intermediate increments of 0.05mL (= 0.05mg captopril)
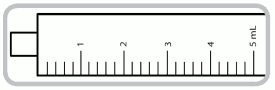
- 5mL syringe graduated with numbered increments of 1mL (= 1mg captopril) and intermediate increments of 0.2mL (= 0.2mg captopril).
The 25mg/5ml product is supplied with the following administration devices:
- 5mL syringe graduated with numbered increments of 1mL (= 5mg captopril) and intermediate increments of 0.2mL (= 1mg captopril).
- 30 mL measuring cup graduated in numbered increments of 5 mL (= 25mg captopril) and intermediate increments of 1mL (= 5mg captopril).
Dose should be individualized according to patient’s profile (see section 4.4) and blood pressure response. The recommended maximum daily dose is 150 mg.
Noyada oral solution may be taken before, during and after meals.
Hypertension: the recommended starting dose is 25-50 mg daily in two divided doses. The dose may be increased incrementally, with intervals of at least 2 weeks, to 100-150 mg/day in two divided doses as needed to reach target blood pressure. Noyada oral solution may be used alone or with other antihypertensive agents, especially thiazide diuretics (see sections 4.3, 4.4, 4.5 and 5.1). A once-daily dosing regimen may be appropriate when concomitant antihypertensive medication such as thiazide diuretics is added.
In patients with a strongly active renin-angiotensin-aldosterone system (hypovolaemia, renovascular hypertension, cardiac decompensation) it is preferable to commence with a single dose of 6.25 mg or 12.5 mg. The inauguration of this treatment should preferably take place under close medical supervision. These doses will then be administered at a rate of two per day. The dosage can be gradually increased to 50 mg per day in one or two doses and if necessary to 100 mg per day in one or two doses.
Heart failure: treatment with captopril for heart failure should be initiated under close medical supervision. The usual starting dose is 6.25 mg – 12.5 mg BID or TID. Titration to the maintenance dose (75 – 150 mg per day) should be carried out based on patient’s response, clinical status and tolerability, up to a maximum of 150 mg per day in divided doses. The dose should be increased incrementally, with intervals of at least 2 weeks to evaluate patient’s response.
Myocardial infarction:
- short-term treatment: Captopril treatment should begin in hospital as soon as possible following the appearance of the signs and/or symptoms in patients with stable haemodynamics. A 6.25 mg test dose should be administered, with a 12.5 mg dose being administered 2 hours afterwards and a 25 mg dose 12 hours later. From the following day, captopril should be administered in a 100 mg/day dose, in two daily administrations, for 4 weeks, if warranted by the absence of adverse haemodynamic reactions. At the end of the 4 weeks of treatment, the patient’s state should be reassessed before a decision is taken concerning treatment for the post-myocardial infarction stage.
- chronic treatment: if captopril treatment has not begun during the first 24 hours of the acute myocardial infarction stage, it is suggested that treatment be instigated between the 3rd and 16th day post-infarction once the necessary treatment conditions have been attained (stable haemodynamics and management of any residual ischaemia). Treatment should be started in hospital under strict surveillance (particularly of blood pressure) until the 75 mg dose is reached. The initial dose must be low (see section 4.4), particularly if the patient exhibits normal or low blood pressure at the initiation of therapy. Treatment should be initiated with a dose of 6.25 mg followed by 12.5 mg 3 times daily for 2 days and then 25 mg 3 times daily if warranted by the absence of adverse haemodynamic reactions. The recommended dose for effective cardioprotection during long-term treatment is 75 to 150 mg daily in two or three doses. In cases of symptomatic hypotension, as in heart failure, the dosage of diuretics and/or other concomitant vasodilators may be reduced in order to attain the steady state dose of captopril. Where necessary, the dose of captopril should be adjusted in accordance with the patient’s clinical reactions. Captopril may be used in combination with other treatments for myocardial infarction such as thrombolytic agents, beta-blockers and acetylsalicylic acid.
Type I Diabetic nephropathy: in patients with type I diabetic nephropathy, the recommended daily dose of captopril is 50-100 mg in two or three divided doses. If additional lowering of blood pressure is desired, additional antihypertensive medications may be added (see sections 4.3, 4.4, 4.5 and 5.1).
Renal impairment: since captopril is excreted primarily via the kidneys, dosage should be reduced or the dosage interval should be increased in patients with impaired renal function. When concomitant diuretic therapy is required, a loop diuretic (e.g. furosemide), rather than a thiazide diuretic, is preferred in patients with severe renal impairment.
In patients with impaired renal function, the following daily dose may be recommended to avoid accumulation of captopril.
| Creatinine clearance (ml/min/1.73 m²) | Daily starting dose (mg) | Daily maximum dose (mg) |
|---|---|---|
| >40 | 25-50 | 150 |
| 21-40 | 25 | 100 |
| 10-20 | 12.5 | 75 |
| <10 | 6.25 | 37.5 |
Elderly patients: as with other antihypertensive agents, consideration should be given to initiating therapy with a lower starting dose (6.25 mg BID) in elderly patients who may have reduced renal function and other organ dysfunctions (see above and section 4.4).
Dosage should be titrated against the blood pressure response and kept as low as possible to achieve adequate control.
Paediatric Population:
The efficacy and safety of captopril have not been fully established. The use of captopril in children and adolescents should be initiated under close medical supervision. The initial dose of captopril is about 0.3mg/kg body weight to be divided in 3 equal doses daily. For patients requiring special precautions (children with renal dysfunction, premature infants, new-borns and infants, because their renal function is not the same as older children and adults) the starting dose should be only 0.15mg captopril/kg weight. Generally, captopril is administered to children 3 times a day, but dose and interval of dose should be adapted individually according to patient’s response.
Method of administration
For oral use only.
Switching between Noyada and other captopril formulations
Once titrated to an effective dose of Noyada Oral solution, patients should remain on their treatment and re-titration should be performed when changing between Noyada and other captopril formulations.
4.9. Overdose
Symptoms of overdosage are severe hypotension, shock, stupor, bradycardia, electrolyte disturbances and renal failure.
After ingestion of an overdose, the patient should be kept under close supervision, preferably in an intensive care unit. Serum electrolytes and creatinine should be monitored frequently, as well as blood pressure. Therapeutic measures depend on the nature and severity of the symptoms.
Measures to prevent absorption (e.g. gastric lavage, administration of adsorbents and sodium sulphate within 30 minutes after intake) and hasten elimination should be applied if ingestion is recent. If hypotension occurs, the patient should be placed in the shock position and salt and volume supplementations should be given rapidly. Treatment with angiotensin-II should be considered. Bradycardia or extensive vagal reactions should be treated by administering atropine. The use of a pacemaker may be considered.
Captopril may be removed from circulation by haemodialysis. The use of high-flux polyacrylonitrile membranes should be avoided.
6.3. Shelf life
Unopened: 18 months
After first opening: 21 days.
6.4. Special precautions for storage
Do not refrigerate.
Do not store above 25°C.
Store in the outer carton, in order to protect from light.
6.5. Nature and contents of container
mber glass bottle with child resistant and tamper evident caps. Each bottle is packed in a cardboard carton containing 1ml and 5ml syringes with an adaptor along with a patient leaflet.
5 mg/5 ml oral solution.
1 ml syringe- each numbered increment is 0.1 ml equivalent to 0.1 mg of captopril. The intermediate increments are 0.05 ml equivalent to 0.05 mg of captopril.
5 ml syringe- each numbered increment is 1 ml equivalent to 1 mg of captopril. The intermediate increments are 0.2 ml equivalent to 0.2 mg of captopril.
Pack size: 100ml
6.6. Special precautions for disposal and other handling
No special requirements for disposal.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.