OMIDRIA Concentrate for solution for intraocular irrigation Ref.[51721] Active ingredients: Ketorolac Phenylephrine
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Rayner Surgical (Ireland) Limited, The Mill Enterprise Hub, Newtown Link Road, Drogheda, A92 CD3D, Co. Louth, Ireland, Tel +353 (0) 860592303, Fax +44 (0) 1903 751 470, Email GerKemmy@rayner.com
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, Sympathomimetics excl. antiglaucoma preparations
ATC code: S01FB51
Mechanism of action
The phenylephrine and ketorolac in Omidria act by distinct mechanisms, to maintain intraoperative mydriasis, to prevent intraoperative miosis, and to reduce acute postoperative pain.
Phenylephrine is an α1-adrenergic receptor agonist and acts as a mydriatic agent by contracting the radial muscle of the iris, dilating the pupil with little or no cycloplegia. Vasoconstriction occurs in the conjunctival circulation and in other ocular vessels to the extent that they are exposed to medicinal product.
Ketorolac is an NSAID that inhibits both cyclooxygenase enzymes (COX1 and COX2), reducing pain and inflammation by decreasing tissue concentrations of prostaglandins resulting from surgical trauma. Ketorolac, by inhibiting prostaglandin synthesis secondary to ocular surgical insult or direct mechanical stimulation of the iris, may also contribute to the prevention of surgically induced miosis.
Clinical efficacy and safety
The efficacy and safety of Omidria was evaluated in two Phase 3, randomised, multicentre, doublemasked, placebo-controlled clinical studies in 808 adult patients undergoing intraocular lens replacement. The population in the studies was 26 to 90 years of age (59% female, 41% male; 80% white, 12% black and 8% other race). Nineteen percent of cataracts were LOCS II Nuclear Grade 2 or 3. Fifty-three percent of patients had brown irides, 28% had blue irides, and 19% had irides of other colours.
Patients were randomised to either Omidria or placebo (1:1). All patients were treated with standardised preoperative topical mydriatic and anaesthetic agents. Pupil diameter was measured throughout the surgical procedure. Postoperative pain was evaluated by a self-administered 0-100 mm visual analogue scale (VAS).
Statistical tests for the change from baseline in pupil diameter (mm) during surgery were carried out with the Cochran-Mantel-Haenszel (CMH) test adjusted for the randomisation strata. In Study 1, the CMH weighted mean difference (Omidria – placebo) in the mean area under the curve (AUC) was 0.58 mm [95% confidence interval: 0.48, 0.68] (P<0.0001). In Study 2, the CMH weighted mean difference (Omidria – placebo) in the mean AUC was 0.59 mm [95% confidence interval: 0.49, 0.69] (P<0.0001).
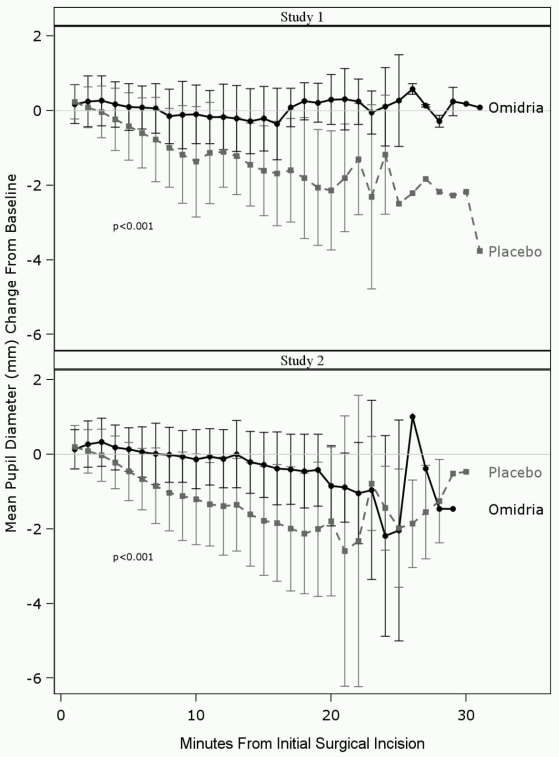
Mydriasis was maintained in the Omidria-treated groups, while the placebo-treated groups experienced progressive constriction of the pupil (see Figure 1.).
Figure 1. Intraoperative pupil diameter (mm) change from baseline:
Prevention of miosis was confirmed in a categorical analysis. In Study 1, only 4% of patients in the Omidria group compared to 23% of patients in the placebo group had a pupil diameter <6 mm at the time of cortical clean-up, and 3% of patients in the Omidria group compared to 28% of patients in the placebo group had a pupil constriction ≥2.5 mm (P<0.0001 in both instances, Chi-Square test). In Study 2, only 4% of patients in the Omidria group compared to 23% of patients in the placebo group had a pupil diameter <6 mm at cortical clean-up, and 1% of patients in the Omidria group compared to 27% of patients in the placebo group had a pupil constriction ≥2.5 mm (P<0.0001, Chi-Square test).
| Placebo | Omidria | |
|---|---|---|
| Study 1 Analysis set (n) | N=201 (n=180) | N=201 (n=184) |
| AUC change from baseline in pupil diameter (mm) during surgery (co-primary endpoint) [mean (SD)] | -0.5 (0.58) | 0.1 (0.41) |
| Diameter <6 mm at any time | 85 (47%) | 19 (10%) |
| Diameter <6 mm at cortical clean-up | 41 (23%) | 7 (4%) |
| ≥2.5 mm pupillary constriction | 50 (28%) | 6 (3%) |
| Study 2 Analysis set (n) | N=204 (n=200) | N=202 (n=195) |
| AUC change from baseline in pupil diameter (mm) during surgery (co-primary endpoint) [mean (SD)] | -0.5 (0.57) | 0.1 (0.43) |
| Diameter <6 mm at any time | 76 (38%) | 18 (9%) |
| Diameter <6 mm at cortical clean-up | 46 (23%) | 8 (4%) |
| ≥2.5 mm pupillary constriction | 53 (27%) | 2 (1%) |
A significant reduction in ocular pain during the initial 10-12 hours postoperatively was also demonstrated. Statistical tests for pain as determined from the 100-mm VAS were carried out with a CMH test adjusted for the randomisation strata. In Study 1, the CMH weighted mean difference (Omidria – placebo) in the mean AUC was -5.20 mm [95% confidence interval: -7.31, -3.09] (P<0.001). In Study 2, the CMH weighted mean difference (Omidria – placebo) in the mean AUC was -4.58 mm [95% confidence interval: -6.92, -2.24] (P<0.001).
| Placebo | Omidria | |
|---|---|---|
| Study 1 Analysis set (n) | N=201 (n=201) | N=201 (n=201) |
| AUC 12 hour ocular pain VAS score (co-primary endpoint) [mean±SD] | 9.2±12.9 | 4.1±8.07 |
| Subjects with VAS = 0 at all times | 28 (14%) | 48 (24%) |
| Subjects with VAS ≥ 40 at any time | 30 (15%) | 13 (7%) |
| Study 2 Analysis set (n) | N=204 (n=202) | N=202 (n=202) |
| AUC 12 hour ocular pain VAS score (co-primary endpoint) [mean±SD] | 8.9±15.19 | 4.3±8.75 |
| Subjects with VAS = 0 at all times | 41 (20%) | 56 (28%) |
| Subjects with VAS ≥ 40 at any time | 27 (13%) | 16 (8%) |
Histologic examination in non-clinical toxicology studies demonstrated no treatment-related effects on the cornea and, in clinical studies with Omidria, no detrimental effects were observed on bestcorrected visual acuity (BCVA). Endothelial cell counts were not conducted during the clinical studies.
Paediatric Population
The European Medicines Agency has deferred the obligation to submit the results of studies with Omidria in one or more subsets of the paediatric population in lens therapeutic procedures (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
In a pharmacokinetic study evaluating Omidria, systemic exposure to both phenylephrine and ketorolac was minimal and transient.
Absorption
Detectable phenylephrine plasma concentrations were observed in only one of 14 patients. The maximum concentration observed in this patient was 1.7 ng/mL, occurring after instillation of topical preoperative phenylephrine drops and prior to exposure to Omidria.
Ketorolac plasma concentrations were detected in 11 of 14 patients. The maximum ketorolac concentration seen was 4.2 ng/mL.
5.3. Preclinical safety data
Non-clinical data reported in the literature for the individual components in Omidria revealed no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
A single-dose toxicology study was conducted in African green monkeys exposed to ocular irrigation solutions containing the combination of phenylehphrine and ketorolac used during lens replacement surgery. No drug-related adverse reactions or pathological findings were observed, with combinations of phenylephrine and ketorolac in irrigation solution administered at concentrations up to 7200 µM phenylephrine and 900 µM ketorolac. These concentrations are over 10-fold higher than the concentration of each agent administered clinically in patients receiving Omidria.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.