PAXLOVID Film-coated tablet Ref.[49623] Active ingredients: Nirmatrelvir Nirmatrelvir and Ritonavir Ritonavir
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets. Nirmatrelvir is a SARS-CoV-2 main protease (Mpro) inhibitor, and ritonavir is an HIV-1 protease inhibitor and CYP3A inhibitor.
Nirmatrelvir
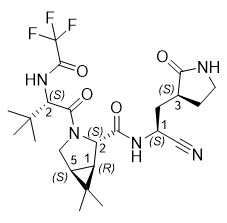
The chemical name of active ingredient of nirmatrelvir is (1R,2S,5S)-N-((1S)-1-Cyano-2-((3S)-2-oxopyrrolidin-3-yl)ethyl)-3-((2S)-3,3-dimethyl-2-(2,2,2-trifluoroacetamido)butanoyl)-6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxamide]. It has a molecular formula of C23H32F3N5O4 and a molecular weight of 499.54.
Nirmatrelvir has the following structural formula:
Nirmatrelvir is available as immediate-release, film-coated tablets. Each tablet contains 150 mg nirmatrelvir with the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, lactose monohydrate, microcrystalline cellulose, and sodium stearyl fumarate. The following are the ingredients in the film coating: hydroxy propyl methylcellulose, iron oxide red, polyethylene glycol, and titanium dioxide.
Ritonavir
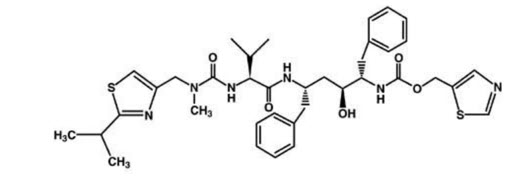
Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1 methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12- tetraazatridecan-13-oic acid, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C37H48N6O5S2, and its molecular weight is 720.95.
Ritonavir has the following structural formula:
Ritonavir is available as film-coated tablets. Each tablet contains 100 mg ritonavir with the following inactive ingredients: anhydrous dibasic calcium phosphate, colloidal silicon dioxide, copovidone, sodium stearyl fumarate, and sorbitan monolaurate. The following are the ingredients in the film coating: colloidal silicon dioxide, hydroxypropyl cellulose, hypromellose, polyethylene glycol 400, polyethylene glycol 3350, polysorbate 80, talc, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets.
|
| How Supplied |
|---|
|
PAXLOVID is nirmatrelvir tablets co-packaged with ritonavir tablets.
Nirmatrelvir tablets and ritonavir tablets are supplied in separate blister cavities within the same child-resistant blister card. Each carton contains 30 tablets divided in 5 daily-dose blister cards (NDC number: 0069-1085-30). Each daily blister card (NDC number: 0069-1085-06) contains 4 nirmatrelvir tablets (150 mg each) and 2 ritonavir tablets (100 mg each) and indicates which tablets need to be taken in the morning and evening. |
Drugs
| Drug | Countries | |
|---|---|---|
| PAXLOVID | Austria, Australia, Canada, Estonia, Spain, Finland, France, Croatia, Ireland, Italy, Japan, Lithuania, New Zealand, Poland, Romania, United Kingdom, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.