PAXLOVID Film-coated tablet Ref.[49623] Active ingredients: Nirmatrelvir Nirmatrelvir and Ritonavir Ritonavir
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
Nirmatrelvir is a peptidomimetic inhibitor of the SARS-CoV-2 main protease (Mpro), also referred to as 3C-like protease (3CLpro) or nsp5 protease. Inhibition of SARS-CoV-2 Mpro renders it incapable of processing polyprotein precursors, preventing viral replication. Nirmatrelvir inhibited the activity of recombinant SARS-CoV-2 Mpro in a biochemical assay with a Ki value of 3.1 nM and an IC50 value of 19.2 nM. Nirmatrelvir was found to bind directly to the SARS-CoV-2 Mpro active site by X-ray crystallography.
Ritonavir is an HIV-1 protease inhibitor but is not active against SARS-CoV-2 Mpro. Ritonavir inhibits the CYP3A-mediated metabolism of nirmatrelvir, resulting in increased plasma concentrations of nirmatrelvir.
12.3. Pharmacokinetics
The pharmacokinetics of nirmatrelvir/ritonavir have been studied in healthy subjects.
Ritonavir is administered with nirmatrelvir as a pharmacokinetic enhancer resulting in higher systemic concentrations and longer half-life of nirmatrelvir, thereby supporting a twice daily administration regimen.
Upon oral administration of nirmatrelvir/ritonavir, the increase in systemic exposure appears to be less than dose proportional up to 750 mg as a single dose and up to 500 mg twice daily as multiple doses. Twice daily dosing over 10 days achieved steady-state on Day 2 with approximately 2-fold accumulation. The pharmacokinetic properties of nirmatrelvir/ritonavir are displayed in Table 2.
Table 2. Pharmacokinetic Properties of Nirmatrelvir and Ritonavir in Healthy Subjects:
| Nirmatrelvir (When Given With Ritonavir) | Ritonavir | |
|---|---|---|
| Absorption | ||
| Tmax (h), median | 3.00* | 3.98* |
| Distribution | ||
| % bound to human plasma proteins | 69% | 98–99% |
| Blood-to-plasma ratio | 0.60 | 0.14† |
| Vz/F (L), mean | 104.7‡ | 112.4‡ |
| Elimination | ||
| Major route of elimination | Renal elimination§ | Hepatic metabolism |
| Half-life (t1/2) (hr), mean | 6.05* | 6.15* |
| Oral clearance (CL/F), mean | 8.99‡ | 13.92‡ |
| Metabolism | ||
| Metabolic pathways | Minimal§ | Major CYP3A4, Minor CYP2D6 |
| Excretion | ||
| % drug-related material in feces | 49.6%¶ | 86.4%# |
| % drug-related material in urine | 35.3%¶ | 11.3%# |
* Represents data after a single dose of 300 mg nirmatrelvir (2 × 150 mg tablet formulation) administered together with 100 mg ritonavir tablet in healthy subjects.
† Red blood cell to plasma ratio.
‡ 300 mg nirmatrelvir (oral suspension formulation) and 100 mg ritonavir (tablet formulation) administered together twice a day for 3 days.
§ Nirmatrelvir is a CYP3A4 substrate but when dosed with ritonavir metabolic clearance is minimal.
¶ Determined by 19F-NMR analysis following 300 mg oral suspension
enhanced with 100 mg ritonavir at -12 hours, 0 hours, 12 hours, and 24 hours.
# Determined by C analysis following 600 mg 14C-ritonavir oral solution.
Single dose pharmacokinetic data of PAXLOVID in healthy subjects is depicted below (Table 3).
Table 3. Single Dose Pharmacokinetics of Nirmatrelvir Following Dosing with 300 mg/100 mg Nirmatrelvir/Ritonavir in Healthy Subjects:
| PK Parameter (units) | Nirmatrelvir (N=12) |
|---|---|
| Cmax (µg/mL) | 2.21 (33) |
| AUCinf (µg*hr/mL) | 23.01 (23) |
| Tmax (hr) | 3.00 (1.02–6.00) |
| T1/2 (hr) | 6.05 ± 1.79 |
Represents data from 2 × 150 mg tablets of nirmatrelvir. Values are presented as geometric mean (geometric % CV) except median (range) for Tmax and arithmetic mean ± SD for T1/2.
Effect of Food on Oral Absorption of Nirmatrelvir
Dosing with a high fat meal modestly increased the exposure of nirmatrelvir (approximately 15% increase in mean Cmax and 1.6% increase in mean AUClast) relative to fasting conditions following administration of a suspension formulation of nirmatrelvir co-administered with ritonavir tablets.
Specific Populations
The pharmacokinetics of nirmatrelvir/ritonavir based on age and gender have not been evaluated.
Pediatric Patients
The pharmacokinetics of nirmatrelvir/ritonavir in patients less than 18 years of age have not been evaluated.
Using a population PK model, the dosing regimen is expected to result in comparable steady-state plasma exposure of nirmatrelvir in patients 12 years of age and older and weighing at least 40 kg to those observed in adults after adjusting for body weight.
Racial or Ethnic Groups
Systemic exposure in Japanese subjects was numerically lower but not clinically meaningfully different than those in Western subjects.
Patients with Renal Impairment
An open-label study compared nirmatrelvir/ritonavir pharmacokinetics in healthy adult subjects and subjects with mild (eGFR ≥60 to <90 mL/min), moderate (eGFR ≥30 to <60 mL/min), and severe (eGFR <30 mL/min) renal impairment following administration of a single oral dose of nirmatrelvir 100 mg enhanced with ritonavir 100 mg administered at -12, 0, 12, and 24 hours. Compared to healthy controls with no renal impairment, the Cmax and AUC of nirmatrelvir in patients with mild renal impairment was 30% and 24% higher, in patients with moderate renal impairment was 38% and 87% higher, and in patients with severe renal impairment was 48% and 204% higher, respectively (Table 4).
Table 4: Impact of Renal Impairment on Nirmatrelvir/Ritonavir Pharmacokinetics
| Normal Renal Function (n=8) | Mild Renal Impairment (n=8) | Moderate Renal Impairment (n=8) | Severe Renal Impairment (n=8) | |
|---|---|---|---|---|
| Cmax (µg/mL) | 1.60 (31) | 2.08 (29) | 2.21 (17) | 2.37 (38) |
| AUCinf (µg*hr/mL) | 14.46 (20) | 17.91 (30) | 27.11 (27) | 44.04 (33) |
| Tmax (hr) | 2.0 (1.0–4.0) | 2.0 (1.0–3.0) | 2.50 (1.0–6.0) | 3.0 (1.0–6.1) |
| T1/2 (hr) | 7.73 ± 1.82 | 6.60 ± 1.53 | 9.95 ± 3.42 | 13.37 ± 3.32 |
Values are presented as geometric mean (geometric % CV) except median (range) for Tmax and arithmetic mean ± SD for t1/2.
Patients with Hepatic Impairment
A single oral dose of 100 mg nirmatrelvir enhanced with 100 mg ritonavir at -12 hours, 0 hours, 12 hours and 24 hours in subjects with moderate hepatic impairment resulted in similar exposures compared to subjects with normal hepatic function (Table 5).
Table 5. Impact of Hepatic Impairment on Nirmatrelvir/Ritonavir Pharmacokinetics:
| Normal Hepatic Function (n=8) | Moderate Hepatic Impairment (n=8) | |
|---|---|---|
| Cmax (µg/mL) | 1.89 (20) | 1.92 (48) |
| AUCinf (µg*hr/mL) | 15.24 (36) | 15.06 (43) |
| Tmax (hr) | 2.0 (0.6–2.1) | 1.5 (1.0–2.0) |
| T1/2 (hr) | 7.21 ± 2.10 | 5.45 ± 1.57 |
Values are presented as geometric mean (geometric % CV) except median (range) for Tmax and arithmetic mean ± SD for t1/2.
Nirmatrelvir/ritonavir has not been studied in patients with severe hepatic impairment.
Drug Interaction Studies Conducted with Nirmatrelvir
In vitro data indicates that nirmatrelvir is a substrate for human MDR1 (P-gp) and 3A4, but not a substrate for human BCRP, MATE1, MATE2K, NTCP, OAT1, OAT2, OAT3, OCT1, OCT2, PEPT1, OATPs 1B1, 1B3, 2B1, or 4C1.
Nirmatrelvir does not reversibly inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2D6 in vitro at clinically relevant concentrations. Nirmatrelvir has the potential to reversibly and time-dependently inhibit CYP3A4 and inhibit MDR1 (P-gp).
Nirmatrelvir does not induce any CYPs at clinically relevant concentrations.
Drug Interaction Studies Conducted with Ritonavir
In vitro studies indicate that ritonavir is mainly a substrate of CYP3A. Ritonavir also appears to be a substrate of CYP2D6 which contributes to the formation of isopropylthiazole oxidation metabolite M-2.
Ritonavir is an inhibitor of CYP3A and to a lesser extent CYP2D6. Ritonavir appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6 as well as other enzymes, including glucuronosyl transferase.
The effects of co-administration of PAXLOVID with itraconazole (CYP3A inhibitor) and carbamazepine (CYP3A inducer) on the nirmatrelvir AUC and Cmax are summarized in Table 6 (effect of other drugs on nirmatrelvir).
Table 6. Drug Interactions: Pharmacokinetic Parameters for Nirmatrelvir in the Presence of the Co-administered Drugs:
| Co-administered Drug | Dose (Schedule) | N | Ratio (in combination with Co-administered drug/alone) of Nirmatrelvir Pharmacokinetic Parameters (90% CI); No Effect=100 | ||
|---|---|---|---|---|---|
| Co-administered Drug | Nirmatrelvir/Ritonavir | Cmax | AUC* | ||
| Carbamazepine† | 300 mg twice daily (16 doses) | 300 mg/100 mg twice daily (5 doses) | 9 | 56.82 (47.04, 68.62) | 44.50 (33.77, 58.65) |
| Itraconazole | 200 mg once daily (8 doses) | 300 mg/100 mg twice daily (5 doses) | 11 | 118.57 (112.50, 124.97) | 138.82 (129.25, 149.11) |
Abbreviations: AUC=area under the plasma concentration-time curve; CI=confidence interval; Cmax=maximum plasma concentrations.
* For carbamazepine, AUC=AUCinf, for itraconazole, AUC=AUCtau.
† Carbamazepine titrated up to 300 mg twice daily on Day 8 through Day 15 (e.g., 100 mg twice daily on Day 1 through Day 3 and 200 mg twice daily on Day 4 through Day 7).
12.4. Microbiology
Antiviral Activity
Nirmatrelvir exhibited antiviral activity against SARS-CoV-2 (USA-WA1/2020 isolate) infection of differentiated normal human bronchial epithelial (dNHBE) cells with EC50 and EC90 values of 62 nM and 181 nM, respectively, after 3 days of drug exposure.
Nirmatrelvir had similar cell culture antiviral activity (EC50 values ≤3-fold relative to USA-WA1/2020) against SARS-CoV-2 isolates belonging to the Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), Delta (B.1.617.2), and Lambda (C.37) variants. The Beta (B.1.351) variant was the least susceptible tested variant with approximately 3-fold reduced susceptibility relative to the USA-WA1/2020 isolate.
No data are available regarding the activity of nirmatrelvir against the SARS-CoV-2 Omicron (B.1.1.529) variant in cell culture. However, in a biochemical assay, the Mpro P132H substitution found in the Omicron variant did not reduce nirmatrelvir activity (Ki fold change <1) compared to the USA-WA1/2020 enzyme.
Antiviral Activity Against SARS-CoV-2 in Animal Models
Nirmatrelvir showed antiviral activity in BALB/c and 129 mice infected with mouse-adapted SARS-CoV-2. Oral administration of nirmatrelvir at 300 mg/kg or 1,000 mg/kg twice daily initiated 4 hours post-inoculation or 1,000 mg/kg twice daily initiated 12 hours post-inoculation resulted in reduction of lung viral titers and ameliorated indicators of disease (weight loss and lung pathology) compared to placebo-treated animals.
Antiviral Resistance
Phenotypic assessments were conducted to characterize the impact of naturally occurring SARS-CoV-2 Mpro polymorphisms on the activity of nirmatrelvir in a biochemical assay using recombinant Mpro enzyme. The clinical significance of these polymorphisms is unknown, and it is also unknown if results from the biochemical assay are predictive of antiviral activity in cell culture. The following Mpro amino acid substitutions were associated with reduced nirmatrelvir activity (≥3-fold higher Ki values): G15S (4.4-fold), T135I (3.5-fold), S144A (91.9-fold), H164N (6.4-fold), H172Y (233-fold), Q189K (65.4-fold), and D248E (3.7-fold). G15S is present in the Lambda variant, which did not have reduced susceptibility to nirmatrelvir (relative to USA-WA1/2020) in cell culture.
In addition, three SARS-CoV-2 Mpro amino acid positions where polymorphisms have not been naturally observed were evaluated by substituting alanine at these positions and assessing their impact on activity in biochemical assays. These Mpro amino acid substitutions were associated with reduced nirmatrelvir activity (i.e., higher Ki values): Y54A (23.6-fold), F140A (39.0-fold), and E166A (33.4-fold). The clinical significance of substitutions at these Mpro positions is unknown.
Cell culture resistance selection studies with nirmatrelvir using mouse hepatitis virus (MHV, a betacoronavirus used as a surrogate) resulted in the emergence of Mpro amino acid substitutions P15A, T50K, P55L, T129M, and/or S144A. The clinical relevance of these changes is not known. The presence of the substitutions P55L and S144A was associated with reduced nirmatrelvir susceptibility (~4- to 5-fold higher EC50 values). These positions correspond to E55 and S144 in SARS-CoV-2 Mpro, respectively. E55L alone did not affect nirmatrelvir activity against SARS-CoV-2 Mpro in a biochemical assay, while S144A reduced nirmatrelvir activity by 91.9-fold (based on Ki value).
Limited SARS-CoV-2 sequencing data are available to characterize nirmatrelvir resistance in clinical trials. The SARS-CoV-2 Mpro substitutions A260V (n=3) or A260T (n=1) emerged in 4% (4/97) of nirmatrelvir/ritonavir treated subjects in clinical trial EPIC-HR with available sequence analysis data. A260T and A260V substitutions are infrequent natural polymorphisms in publicly available SARS-CoV-2 sequences (as of Dec 5, 2021). In a biochemical assay, the A260V Mpro substitution did not reduce nirmatrelvir activity (Ki fold-change <1).
Cross-resistance is not expected between nirmatrelvir and anti-SARS-CoV-2 monoclonal antibodies or remdesivir based on their different mechanisms of action.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Nirmatrelvir
Carcinogenicity studies have not been conducted with nirmatrelvir.
Nirmatrelvir was negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the in vitro micronucleus assay using human lymphoblastoid TK6 cells, and the in vivo rat micronucleus assays.
In a fertility and early embryonic development study, nirmatrelvir was administered orally to male and female rats at doses of 60, 200, or 1,000 mg/kg/day once daily beginning 14 days prior to mating, throughout the mating phase, and continued through GD 6 for females and for a total of 32 doses for males. There were no effects on fertility, reproductive performance, or early embryonic development at doses up to 1,000 mg/kg/day, resulting in systemic exposure (AUC24) approximately 4 times higher than exposure at the authorized human dose of PAXLOVID.
Ritonavir
Carcinogenicity studies in mice and rats have been conducted on ritonavir. In male mice, at levels of 50, 100, or 200 mg/kg/day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 2 times higher (in males) than the exposure in humans at the authorized human dose of PAXLOVID. There were no carcinogenic effects seen in females at the dosages tested. The exposure at the high dose was approximately 4 times higher (in females) than the exposure in humans at the authorized human dose of PAXLOVID. In rats dosed at levels of 7, 15, or 30 mg/kg/day, there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 36% that of the exposure in humans at the authorized human dose of PAXLOVID.
Ritonavir was found to be negative for mutagenic or clastogenic activity in a battery of in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Ritonavir produced no effects on fertility in rats at drug exposures approximately 2 (male) and 4 (female) times higher than the exposure in humans at the authorized human dose of PAXLOVID.
13.2. Animal Toxicology and/or Pharmacology
Studies with nirmatrelvir included repeat dose toxicity studies in rats (14 days) and monkeys (15 days). Repeated daily oral dosing in rats at up to 1,000 mg/kg/day resulted in non-adverse hematological, liver, and thyroid effects. All of the hematology and coagulation findings (i.e., increases in PT and APTT) had no clinical or microscopic correlates and all findings completely recovered at the end of the 2-week recovery period. The liver (i.e., minimal to mild periportal hepatocyte hypertrophy and vacuolation) and thyroid gland (i.e., thyroid follicular cell hypertrophy) findings were consistent with secondary adaptive effects related to microsomal enzyme-induced increase in thyroid hormone clearance in the liver, a mechanism that rats are known to be particularly sensitive to relative to humans. All of the findings observed in the liver and thyroid were low severity and occurred in the absence of correlating alterations in clinical pathology parameters, and all of these findings fully recovered. No adverse effects were observed at doses up to 1,000 mg/kg/day, resulting in systemic exposure approximately 4 times higher than exposures at the authorized human dose of PAXLOVID. Nirmatrelvir-related findings following repeat oral dosing in monkeys for 15 days were limited to emesis and increase in fibrinogen. Increased fibrinogen may be attributed to an inflammatory state but lacked a microscopic correlate. At the high dose of 600 mg/kg/day, the systemic exposure in monkeys was about 18 times higher than exposures at the authorized human dose of PAXLOVID.
14. Clinical Studies
14.1 Efficacy in Subjects at High Risk of Progressing to Severe COVID-19 Illness
The data supporting this EUA are based on the analysis of EPIC-HR (NCT04960202), a Phase 2/3, randomized, double-blind, placebo-controlled study in non-hospitalized symptomatic adult subjects with a laboratory confirmed diagnosis of SARS-CoV-2 infection. Eligible subjects were 18 years of age and older with at least 1 of the following risk factors for progression to severe disease: diabetes, overweight (BMI >25), chronic lung disease (including asthma), chronic kidney disease, current smoker, immunosuppressive disease or immunosuppressive treatment, cardiovascular disease, hypertension, sickle cell disease, neurodevelopmental disorders, active cancer, medically-related technological dependence, or were 60 years of age and older regardless of comorbidities. Subjects with COVID-19 symptom onset of ≤5 days were included in the study. Subjects were randomized (1:1) to receive PAXLOVID (nirmatrelvir/ritonavir 300 mg/100 mg) or placebo orally every 12 hours for 5 days. The study excluded individuals with a history of prior COVID-19 infection or vaccination. The primary efficacy endpoint was the proportion of subjects with COVID-19 related hospitalization or death from any cause through Day 28. The analysis was conducted in the modified intent-to-treat (mITT) analysis set (all treated subjects with onset of symptoms ≤3 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment), the mITT1 analysis set (all treated subjects with onset of symptoms ≤5 days who at baseline did not receive nor were expected to receive COVID-19 therapeutic mAb treatment), and the mITT2 analysis set (all treated subjects with onset of symptoms ≤5 days).
A total of 2,246 subjects were randomized to receive either PAXLOVID or placebo. At baseline, mean age was 46 years; 51% were male; 72% were White, 5% were Black, and 14% were Asian; 45% were Hispanic or Latino; 66% of subjects had onset of symptoms ≤3 days from initiation of study treatment; 47% of subjects were serological negative at baseline; the mean (SD) baseline viral load was 4.63 log10 copies/mL (2.87); 26% of subjects had a baseline viral load of >10^7 (units); 6% of subjects either received or were expected to receive COVID-19 therapeutic monoclonal antibody treatment at the time of randomization and were excluded from the mITT and mITT1 analyses.
The baseline demographic and disease characteristics were balanced between the PAXLOVID and placebo groups.
Table 7 provides results of the primary endpoint in mITT1 analysis population. For the primary endpoint, the relative risk reduction in the mITT1 analysis population for PAXLOVID compared to placebo was 88% (95% CI: 75%, 94%).
Table 7. Efficacy Results in Non-Hospitalized Adults with COVID-19 Dosed within 5 Days of Symptom Onset who Did Not Receive COVID-19 Monoclonal Antibody Treatment at Baseline (mITT1 Analysis Set):
| PAXLOVID (N=1,039) | Placebo (N=1,046) | |
|---|---|---|
| COVID-19 related hospitalization or death from any cause through Day 28 | ||
| n (%) | 8 (0.8%) | 66 (6.3%) |
| Reduction relative to placebo* [95% CI], % | -5.62 (-7.21, -4.03) | |
| All-cause mortality through Day 28, % | 0 | 12 (1.1%) |
Abbreviations: CI=confidence interval.
The determination of primary efficacy was based on a planned interim analysis of 780 subjects in mITT population. The estimated risk reduction was -6.3% with a 95% CI of (-9.0%, -3.6%) and 2-sided p-value <0.0001.
* The estimated cumulative proportion of participants hospitalized or death by Day 28 was calculated for each treatment group using the Kaplan-Meier method, where subjects without hospitalization and death status through Day 28 were censored at the time of study discontinuation.
Consistent results were observed in the mITT and mITT2 analysis populations. A total of 1,379 subjects were included in the mITT analysis population. The event rates were 5/697 (0.72%) in the PAXLOVID group, and 44/682 (6.45%) in the placebo group. The primary SARS-CoV-2 variant across both treatment arms was Delta (98%), including clades 21J, 21A, and 21I.
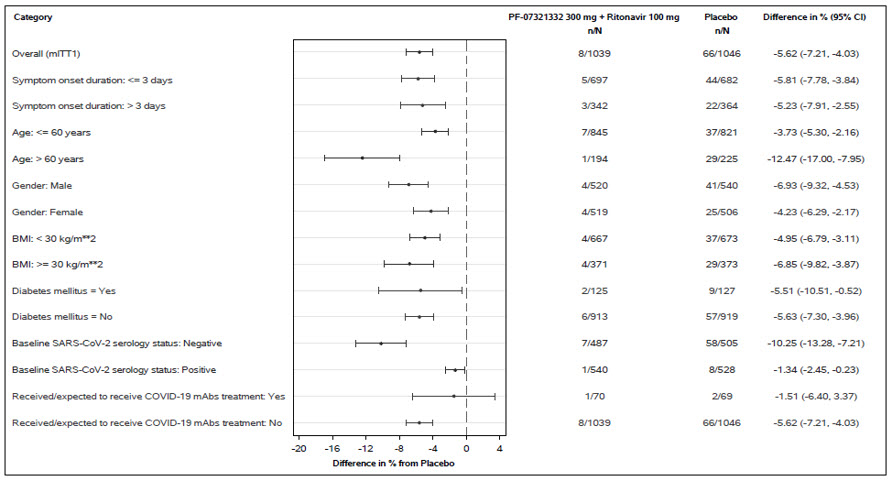
Similar trends have been observed across subgroups of subjects (see Figure 1). These subgroup analyses are considered exploratory.
Figure 1: Adults with COVID-19 Dosed within 5 Days of Symptom Onset with COVID-19-Related Hospitalization or Death from Any Cause Through Day 28 (Protocol C4671005)
N=number of participants in the category of the analysis set.
All categories are based on mITT1 population except for COVID-19 mAb treatment which is based on mITT2 population.
Seropositivity was defined if results were positive in either Elecsys anti-SARS-CoV-2 S or Elecsys anti-SARS-CoV-2 (N) assay.
The difference of the proportions in the 2 treatment groups and its 95% confidence interval based on Normal approximation of the data are presented.
Relative to placebo, PAXLOVID treatment was associated with an approximately 0.9 log10 copies/mL greater decline in viral RNA levels in nasopharyngeal samples through Day 5, with similar results observed in the mITT, mITT1, and mITT2 analysis populations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.