PROLASTIN-C Powder for solution for injection Ref.[27444] Active ingredients: Alfa1 antitrypsin
Source: FDA, National Drug Code (US) Revision Year: 2020
1. Indications and Usage
PROLASTIN-C is an Alpha1-Proteinase Inhibitor (Human) (Alpha1-PI) indicated for chronic augmentation and maintenance therapy in adults with clinical evidence of emphysema due to severe hereditary deficiency of Alpha1-PI (alpha1-antitrypsin deficiency).
PROLASTIN-C increases antigenic and functional (anti-neutrophil elastase capacity, ANEC) serum levels and antigenic lung epithelial lining fluid levels of Alpha1-PI.
Limitations of Use
- The effect of augmentation therapy with any Alpha1-PI, including PROLASTIN-C, on pulmonary exacerbations and on the progression of emphysema in Alpha1-PI deficiency has not been conclusively demonstrated in randomized, controlled clinical trials.
- Clinical data demonstrating the long-term effects of chronic augmentation or maintenance therapy with PROLASTIN-C are not available.
- PROLASTIN-C is not indicated as therapy for lung disease in patients in whom severe Alpha1-PI deficiency has not been established.
2. Dosage and Administration
For intravenous use after reconstitution only.
2.1 Dose
- The recommended dose of PROLASTIN-C is 60 mg/kg body weight administered intravenously once weekly.
- Dose ranging studies using efficacy endpoints have not been performed with any Alpha1-PI product.
- The carton and the label on each vial of PROLASTIN-C show the actual amount of functionally active Alpha1-PI in milligrams (as determined by the capacity to neutralize porcine pancreatic elastase).
2.2 Preparation and Reconstitution
- Allow unopened PROLASTIN-C and diluent vials to warm up to room temperature before reconstitution.
- Remove the plastic flip tops from each vial.
- Swab the exposed stopper surfaces with alcohol and allow to dry.
- Remove the plastic cover from the short end of the transfer needle. Insert the exposed end of the needle through the center of the stopper in the diluent vial.
- Remove the cover at the other end of the transfer needle by twisting it carefully.
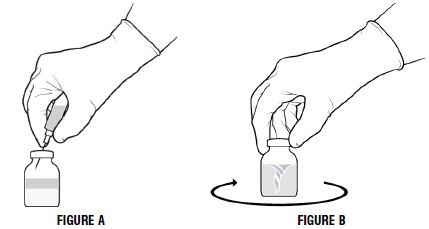
- Invert the diluent vial and insert the attached needle into the PROLASTIN-C vial at a 45° angle (Figure A below). This will direct the stream of diluent against the wall of the product vial and minimize foaming. The vacuum will draw the diluent into the PROLASTIN-C vial.
- Remove the diluent vial and transfer needle.
- Immediately after adding the diluent, swirl vigorously for 10-15 seconds to thoroughly break up cake, then swirl continuously until the powder is completely dissolved (Figure B below). Some foaming will occur, but does not affect the quality of the product.
- Inspect the reconstituted PROLASTIN-C visually for particulate matter and discoloration prior to pooling. A few small particles may remain after reconstitution. If particles are visible, remove by passage through a sterile filter, such as a 15 micron filter used for administering blood products (not supplied).
- Pool reconstituted PROLASTIN-C from several vials into an empty, sterile intravenous solution container using aseptic technique. Use the sterile filter needle provided for this purpose.
- Keep reconstituted solution at room temperature for administration within three hours.
2.3 Administration
- Visually inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Infuse PROLASTIN-C separately, without mixing with other agents or diluting solutions.
- Infuse PROLASTIN-C intravenously at 0.08 mL/kg/min as determined by patient response and comfort. The recommended dosage of 60 mg/kg takes approximately 15 minutes to infuse.
16.2. Storage and Handling
- Store PROLASTIN-C at temperatures not to exceed 25°C (77°F) for the period indicated by the expiration date on its label.
- Avoid freezing as breakage of the diluent bottle might occur.
- Inform patients of the signs of hypersensitivity reactions including pruritus; generalized urticaria; flushing; swollen lips, tongue, or uvula; wheezing; tightness of the chest; dyspnea; hypotension; and syncope. Advise patients to discontinue use of the product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if these symptoms occur [see Warnings and Precautions (5.1)].
- Inform patients that PROLASTIN-C is made from human plasma and may carry a risk of transmitting infectious agents that can cause disease (e.g., viruses, the vCJD agent and, theoretically, the CJD agent). Explain that the risk of PROLASTIN-C transmitting an infectious agent has been reduced by screening plasma donors for prior exposure to certain infectious agents, by testing the donated plasma for certain current virus infections, and by inactivating and/or removing infectious agents during manufacturing [see Warnings and Precautions (5.2 )].
- Inform patients that administration of PROLASTIN-C has been demonstrated to raise the plasma level of Alpha1-PI, but that the effect of this augmentation on pulmonary exacerbations and on the rate of progression of emphysema has not been demonstrated in adequately powered, randomized, controlled clinical trials for any Alpha1-PI product [see Clinical Studies (14)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.