PYLERA Capsule Ref.[50501] Active ingredients: Bismuth subcitrate Methronidazole Tetracycline
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
PYLERA capsules are a combination antimicrobial product containing bismuth subcitrate potassium, metronidazole, and tetracycline hydrochloride for oral administration. Each size 0 elongated capsule contains:
- bismuth subcitrate potassium, 140 mg
- metronidazole, 125 mg
- smaller capsule (size 3) containing tetracycline hydrochloride, 125 mg
Tetracycline hydrochloride is encapsulated within a smaller capsule to create a barrier to avoid contact with bismuth subcitrate potassium.
Each PYLERA capsule contains the following inactive ingredients: Magnesium Stearate NF, Lactose Monohydrate NF, Talc USP, Gelatin USP, and Titanium Dioxide NF, Printed in red ink.
Bismuth subcitrate potassium is a white or almost white powder. It is a soluble, complex bismuth salt of citric acid. The schematized empirical molecular formula of bismuth subcitrate potassium is Bi (Citrate)2K5●3 H2O. The equivalent theoretical molecular formula is BiC12H14K5O17. The molecular mass of the theoretical molecular formula of a single unit of bismuth subcitrate potassium is 834.71.
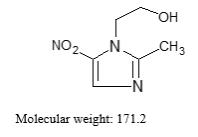
Metronidazole is a white to pale yellow crystalline powder. Metronidazole is 2-methyl-5-nitroimidazole-1-ethanol, with a molecular formula of C6H9N3O3 and the following structural formula:
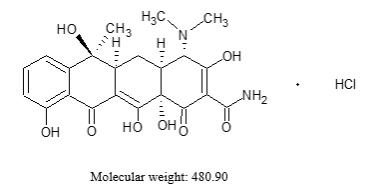
Tetracycline hydrochloride is a yellow, odorless, crystalline powder. Tetracycline hydrochloride is stable in air, but exposure to strong sunlight causes it to darken. Tetracycline hydrochloride is (4S,4aS,5aS,6S,12aS)4(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-penta-hydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide hydrochloride, with a molecular formula of C22H24N2O8●HCl and the following structural formula:
| Dosage Forms and Strengths |
|---|
|
Each PYLERA capsule contains 140 mg of bismuth subcitrate potassium, 125 mg of metronidazole, and a smaller capsule inside containing 125 mg of tetracycline hydrochloride. The capsules are white and opaque, with the APTALISTM logo printed on the body and “BMT” printed on the cap. |
| How Supplied |
|---|
|
PYLERA is supplied as a white opaque capsule containing 140 mg bismuth subcitrate potassium, 125 mg metronidazole, and 125 mg tetracycline hydrochloride, with the APTALISTM logo printed on the body and “BMT” printed on the cap. PYLERA capsules are supplied as bottles of 120 capsules and as the 10 day Therapy pack containing 10 blister cards, with each card containing 12 PYLERA capsules for a total of 120 capsules. NDC Number: 58914-601-21, Bottles of 120. NDC Number: 58914-601-20, Blister pack of 120. Distributed by: Allergan USA, Inc., Madison, NJ 07940 |
Drugs
| Drug | Countries | |
|---|---|---|
| PYLERA | Austria, Estonia, Spain, France, Poland, Tunisia, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.