SAPHRIS Tablet Ref.[27430] Active ingredients: Asenapine
Source: FDA, National Drug Code (US) Revision Year: 2017
Product description
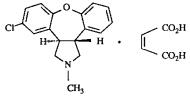
SAPHRIS contains asenapine maleate which is an atypical antipsychotic that is available for sublingual administration. Asenapine belongs to the class dibenzo-oxepino pyrroles. The chemical designation is (3aRS,12bRS)-5-Chloro-2-methyl-2,3,3a,12b-tetrahydro-1Hdibenzo[2,3:6,7]oxepino[4,5-c]pyrrole (2Z)-2-butenedioate (1:1). Its molecular formula is C17H16ClNO•C4H4O4 and its molecular weight is 401.84 (free base: 285.8).
The chemical structure is:
Asenapine maleate is a white to off-white powder.
SAPHRIS, black cherry flavor, is supplied for sublingual administration in tablets containing 2.5 mg, 5 mg or 10 mg asenapine; inactive ingredients include gelatin, mannitol, sucralose, and black cherry flavor.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
SAPHRIS (asenapine) sublingual tablets are supplied as: 2.5 mg Tablets, black cherry flavor: Round, white to off-white sublingual tablets, with a hexagon on one side. Child-resistant packaging: Box of 60 6 blisters with 10 tablets NDC 0456-2402-60 Hospital Unit Dose: Box of 100 10 blisters with 10 tablets NDC 0456-2402-63 5 mg Tablets, black cherry flavor: Round, white to off-white sublingual tablets, with “5” on one side within a circle. Child-resistant packaging: Box of 60 6 blisters with 10 tablets NDC 0456-2405-60 Hospital Unit Dose: Box of 100 10 blisters with 10 tablets NDC 0456-2405-63 10 mg Tablets, black cherry flavor: Round, white to off-white sublingual tablets, with “10” on one side within a circle. Child-resistant packaging: Box of 60 6 blisters with 10 tablets NDC 0456-2410-60 Hospital Unit Dose: Box of 100 10 blisters with 10 tablets NDC 0456-2410-63 Distributed by: Allergan USA, Inc., Irvine, CA 92612 |
Drugs
| Drug | Countries | |
|---|---|---|
| SAPHRIS | Australia, Canada, Israel, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.