SIMBRINZA Eye drops, suspension (eye drops) Ref.[50759] Active ingredients: Brimonidine Brinzolamide
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Novartis Europharm Limited, Vista Building, Elm Park, Merrion Road, Dublin 4, Ireland
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Ophthalmologicals, Antiglaucoma preparations and miotics ATC code: S01EC54
Mechanism of action
SIMBRINZA contains two active substances: brinzolamide and brimonidine tartrate. These two components lower intraocular pressure (IOP) in patients with open-angle glaucoma (OAG) and ocular hypertension (OHT) by suppressing the formation of aqueous humour from the ciliary process in the eye. Although both brinzolamide and brimonidine lower IOP by suppressing aqueous humour formation, their mechanisms of action are different.
Brinzolamide acts by inhibiting the enzyme carbonic anhydrase (CA-II) in the ciliary epithelium that reduces the formation of bicarbonate ions with subsequent reduction in sodium and fluid transport across the ciliary epithelium, resulting in decreased aqueous humour formation. Brimonidine, an alpha-2 adrenergic agonist, inhibits the enzyme adenylate cyclase and suppresses the cAMP-dependent formation of aqueous humour. Additionally, administration of brimonidine results in an increase in uveoscleral outflow.
Pharmacodynamic effects
Clinical efficacy and safety
Monotherapy
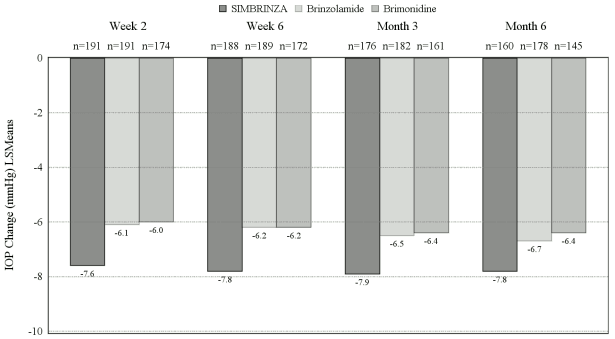
In a 6-month, controlled, contribution of elements clinical study enrolling 560 patients with openangle glaucoma (including pseudoexfoliation or pigment dispersion component) and/or ocular hypertension who, in the investigator’s opinion, were insufficiently controlled on monotherapy or already on multiple IOP-lowering medicinal products, and who had mean baseline diurnal IOP of 26 mmHg, the mean diurnal IOP-lowering effect of SIMBRINZA dosed twice daily was approximately 8 mmHg. Statistically superior reductions in the mean diurnal IOP were observed with SIMBRINZA compared to brinzolamide 10 mg/ml or brimonidine 2 mg/ml dosed twice daily at all visits throughout the study (Figure 1).
Figure 1. Meana diurnal (9 AM, +2 hrs, +7 hrs) IOP change from baseline (mmHg) - Contribution of elements study:
a Least squares means derived from a statistical model that accounts for study site, 9 AM baseline IOP stratum, and correlated IOP measurements within patient. All treatment differences (SIMBRINZA versus individual components) were statistically significant with p=0.0001 or less.
Mean IOP reductions from baseline at each time point at each visit were greater with SIMBRINZA (6 to 9 mmHg) than monotherapy with either brinzolamide (5 to 7 mmHg) or brimonidine (4 to 7 mmHg). Mean percent IOP reductions from baseline with SIMBRINZA ranged from 23 to 34%. The percentages of patients with an IOP measurement less than 18 mmHg were greater in the SIMBRINZA group than in the Brinzolamide group at 11 of 12 assessments through Month 6 and were greater in the SIMBRINZA group than in the Brimonidine group at all 12 assessments through Month 6. At the +2 h time point (the time corresponding to the morning efficacy peak) for the primary efficacy visit at Month 3, the percentage of patients with an IOP less than 18 mmHg was 68.8% in the SIMBRINZA group, 42.3% in the Brinzolamide group, and 44.0% in the Brimonidine group.
In a 6-month, controlled, non-inferiority clinical study enrolling 890 patients with open-angle glaucoma (including pseudoexfoliation or pigment dispersion component) and/or ocular hypertension who, in the investigator’s opinion, were insufficiently controlled on monotherapy or already on multiple IOP-lowering medicinal products, and who had mean baseline diurnal IOP of 26 to 27 mmHg, non-inferiority of SIMBRINZA compared to brinzolamide 10 mg/mL + brimonidine 2 mg/mL dosed concomitantly was demonstrated at all visits throughout the study with respect to mean diurnal IOP reduction from baseline (Table 1).
Table 1. Comparison of mean diurnal IOP (mmHg) change from baseline – Non-inferiority study:
| Visit | SIMBRINZA Meana | Brinzolamide + Brimonidine Meana | Difference Meana (95% CI) |
|---|---|---|---|
| Week 2 | -8.4 (n=394) | -8.4 (n=384) | -0.0 (-0.4, 0.3) |
| Week 6 | -8.5 (n=384) | -8.4 (n=377) | -0.1 (-0.4, 0.2) |
| Month 3 | -8.5 (n=384) | -8.3 (n=373) | -0.1 (-0.5, 0.2) |
| Month 6 | -8.1 (n=346) | -8.2 (n=330) | 0.1 (-0.3, 0.4) |
a Least squares means derived from a statistical model that accounts for study site, 9 AM baseline IOP stratum, and correlated IOP measurements within patient
Mean IOP reductions from baseline at each time point at each visit with SIMBRINZA or the individual components administered concomitantly were similar (7 to 10 mmHg). Mean percent IOP reductions from baseline with SIMBRINZA ranged from 25 to 37%.The percentages of patients with an IOP measurement less than 18 mmHg were similar across study visits for the same time point through Month 6 in the SIMBRINZA and Brinzolamide + Brimonidine groups. At the +2 h time point (the time corresponding to the morning efficacy peak) for the primary efficacy visit at Month 3, the percentage of patients with an IOP less than 18 mmHg was 71.6% in both study groups.
Adjunct therapy
Clinical data on the use of SIMBRINZA adjunctive to prostaglandin analogues (PGA) also showed superior IOP-lowering efficacy of SIMBRINZA + PGA compared with the PGA alone. In study CQVJ499A2401, SIMBRINZA + PGA (i.e. travoprost, latanoprost, or bimatoprost) demonstrated superior IOP-lowering efficacy from baseline compared to Vehicle + PGA after 6 weeks of treatment, with between-treatment difference in model-adjusted mean change from baseline in diurnal IOP of -3.44 mmHg (95% CI, -4.2, -2.7; p-value <0.001).
Clinical data on the use of SIMBRINZA adjunctive to travoprost-timolol maleate fixed dose combination eye drops, solution also showed superior IOP-lowering efficacy of SIMBRINZA + travoprost-timolol maleate eye drops compared with the travoprost-timolol maleate alone. In study CQVJ499A2402, SIMBRINZA + travoprost-timolol maleate eye drops demonstrated superior IOPlowering efficacy from baseline compared to Vehicle + travoprost-timolol maleate eye drops after 6 weeks of treatment, with between-treatment difference in model-adjusted mean change from baseline in diurnal IOP of -2.15 mmHg (95% CI, -2.8, -1.5; p-value <0.001).
The safety profile of SIMBRINZA in adjunct therapy was similar to that observed with SIMBRINZA monotherapy.
There are no efficacy and safety data for adjunct therapy beyond 6 weeks.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with SIMBRINZA in all subsets of the paediatric population in the treatment of glaucoma and ocular hypertension (see section 4.2 for information on paediatric use).
5.2. Pharmacokinetic properties
Absorption
Brinzolamide is absorbed through the cornea following topical ocular administration. The substance is also absorbed into the systemic circulation, where it binds strongly to carbonic anhydrase in red blood cells (RBCs). Plasma concentrations are very low. Whole blood elimination half-life is prolonged (>100 days) in humans due to RBC carbonic anhydrase binding.
Brimonidine is rapidly absorbed into the eye following topical administration. In rabbits, maximum ocular concentrations were achieved in less than one hour in most cases. Maximum human plasma concentrations are <1 ng/mL and achieved within <1 hour. Plasma levels decline with a half-life of approximately 2-3 hours. No accumulation occurs during chronic administration.
In a topical ocular clinical study comparing the systemic pharmacokinetics of SIMBRINZA administered two or three times daily to brinzolamide and brimonidine administered individually using the same two posologies, the steady-state whole blood brinzolamide and N-desethylbrinzolamide pharmacokinetics were similar between the combination product and brinzolamide administered alone. Likewise, the steady-state plasma pharmacokinetics of brimonidine from the combination were similar to those observed for brimonidine administered alone, with the exception of the twice daily SIMBRINZA treatment group, for which the mean AUC0-12 hours was about 25% lower than that for brimonidine alone administered twice daily.
Distribution
Studies in rabbits showed that maximum brinzolamide ocular concentrations following topical administration are in the anterior tissues such as cornea, conjunctiva, aqueous humour and iris-ciliary body. Retention in ocular tissues is prolonged due to binding to carbonic anhydrase. Brinzolamide is moderately (about 60%) bound to human plasma proteins.
Brimonidine exhibits affinity for pigmented ocular tissues, particularly iris-ciliary body, due to its known melanin binding properties. However, clinical and non-clinical safety data show it to be welltolerated and safe during chronic administration.
Biotransformation
Brinzolamide is metabolised by hepatic cytochrome P450 isozymes, specifically CYP3A4, CYP2A6, CYP2B6, CYP2C8 and CYP2C9. The primary metabolite is N-desethylbrinzolamide, followed by the N-desmethoxypropyl and O-desmethyl metabolites, as well as an N-propionic acid analogue formed by oxidation of the N-propyl side chain of O-desmethyl brinzolamide. Brinzolamide and Ndesethylbrinzolamide do not inhibit cytochrome P450 isozymes at concentrations at least 100-fold above maximum systemic levels.
Brimonidine is extensively metabolised by hepatic aldehyde oxidase, with formation of 2- oxobrimonidine, 3-oxobrimonidine and 2,3-dioxobrimonidine being the major metabolites. Oxidative cleavage of the imidazoline ring to 5-bromo-6-guanidinoquinoxaline is also observed.
Elimination
Brinzolamide is primarily eliminated in urine unchanged. In humans, urinary brinzolamide and Ndesethylbrinzolamide accounted for about 60 and 6% of the dose, respectively. Data in rats showed some biliary excretion (about 30%), primarily as metabolites.
Brimonidine is primarily eliminated in the urine as metabolites. In rats and monkeys, urinary metabolites accounted for 60 to 75% of oral or intravenous doses.
Linearity / non-linearity
Brinzolamide pharmacokinetics are inherently non-linear due to saturable binding to carbonic anhydrase in whole blood and various tissues. Steady-state exposure does not increase in a doseproportional manner.
In contrast, brimonidine exhibits linear pharmacokinetics over the clinically therapeutic dose range.
Pharmacokinetic / pharmacodynamic relationship(s)
SIMBRINZA is intended for local action within the eye. Assessment of human ocular exposure at efficacious doses is not feasible. The pharmacokinetic/pharmacodynamic relationship in humans for IOP-lowering has not been established.
Other special populations
Studies to determine the effects of age, race, and renal or hepatic impairment have not been conducted with SIMBRINZA. A study of brinzolamide in Japanese versus non-Japanese subjects showed similar systemic pharmacokinetics between the two groups. In a study of brinzolamide in subjects with renal impairment, a 1.6- to 2.8-fold increase in the systemic exposure to brinzolamide and Ndesethylbrinzolamide between normal and moderately renally-impaired subjects was demonstrated. This increase in steady-state RBC concentrations of substance-related material did not inhibit RBC carbonic anhydrase activity to levels that are associated with systemic side effects. However, the combination product is not recommended for patients with severe renal impairment (creatinine clearance <30 mL/minute).
The Cmax, AUC and elimination half-life of brimonidine are similar in elderly (>65 years of age) subjects compared to young adults. The effects of renal and hepatic impairment on the systemic pharmacokinetics of brimonidine have not been evaluated. Given the low systemic exposure to brimonidine following topical ocular administration, it is expected that changes in plasma exposure would not be clinically relevant.
Paediatric population
The systemic pharmacokinetics of brinzolamide and brimonidine, alone or in combination, in paediatric patients have not been studied.
5.3. Preclinical safety data
Brinzolamide
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, single-dose toxicity, repeated dose toxicity, genotoxicity and carcinogenic potential.
Effects in non-clinical reproduction and development toxicity studies were observed only at exposures considered sufficiently in excess of the maximum human exposure indicating little relevance to clinical use. In rabbits oral, maternally toxic doses of brinzolamide of up to 6 mg/kg/day (261 times the recommended daily clinical dose of 23 µg/kg/day) revealed no effect on foetal development. In rats doses of 18 mg/kg/day (783 times the recommended daily clinical dose), but not 6 mg/kg/day, resulted in slightly reduced ossification of skull and sternebrae of foetuses. These findings were associated with metabolic acidosis with decreased body weight gain in dams and decreased foetal weights. Dose related decreases in foetal weights were observed in pups of dams given 2 to 18 mg/kg/day. During lactation, the no adverse effect level in the offspring was 5 mg/kg/day.
Brimonidine
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.