SUBLOCADE Solution for injection Ref.[27427] Active ingredients: Buprenorphine
Source: FDA, National Drug Code (US) Revision Year: 2021
12.1. Mechanism of Action
SUBLOCADE Injection contains buprenorphine. Buprenorphine is a partial agonist at the mu- opioid receptor and an antagonist at the kappa-opioid receptor.
12.2. Pharmacodynamics
Mu-Opioid Receptor Occupancy and Association With Opioid Blockade
In a Positron Emission Tomography (PET) study with SUBLOCADE in 2 subjects (one subject receiving 200 mg SC injections and one subject receiving 300 mg SC injections) with opioid use disorder, 75 to 92% occupancy of the mu-opioid receptors in the brain was maintained for 28 days following the last dose under steady-state conditions.
The opioid blockade study evaluated the blockade of subjective opioid effects, pharmacokinetics (PK) and safety of SC injections of SUBLOCADE. Stabilization doses of SL buprenorphine prior to injection of SUBLOCADE failed to provide full blockade of subjective effects of hydromorphone 18 mg IM. After SUBLOCADE injections at Weeks 0 and 4, on average, subjective effects of both 6 mg and 18 mg doses of hydromorphone were blocked; however, wide variability was seen across subjects. Complete blockade continued throughout the 8 weeks of observation that followed the 2nd SUBLOCADE injection [see Clinical Studies (14.1)].
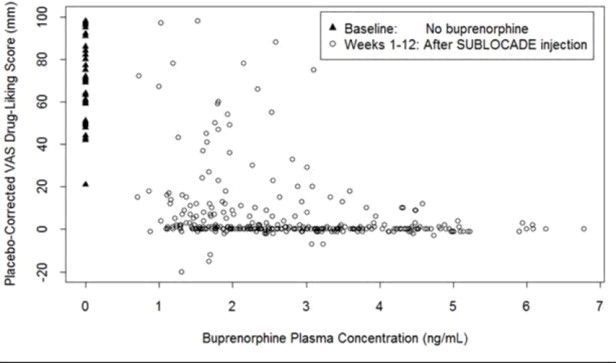
Figure 10 illustrates the relationship between buprenorphine plasma level and drug liking after 18 mg hydromorphone IM.
Figure 10. Drug Liking VAS vs. Plasma Buprenorphine Concentration Following 18 mg Hydromorphone Challenges:
Exposure-response relationships were assessed for illicit opioid use, based on urine samples negative for illicit opioids combined with self-reports negative for illicit opioid use, and withdrawal symptoms using data obtained from 489 opioid dependent patients in the double-blind Phase 3 Study (13-0001).
The observed plateau for maximal response was reached at buprenorphine plasma concentrations of approximately 2-3 ng/mL for illicit opioid use and 4 ng/mL for opioid withdrawal symptoms.
Population PK/PD modeling indicated that patients using opioids by the injectable route at baseline may require higher buprenorphine exposure compared to patients not using opioids by the injectable route at baseline.
Cardiac Electrophysiology
Serial ECGs were collected following a single dose and at steady-state to evaluate the effect of SUBLOCADE on the QT interval in five clinical studies including the Phase 3 study. In a Phase 3 study, seven patients had an increase from baseline QTc greater than 60 msec at any time [2/203 patients (1.0%) in the 300 mg/100 mg group and 5/201 patients (2.0%) in the 300 mg/300 mg group] and one patient in the 300 mg/300 mg group was found to have a QTc greater than 500 msec. These QTc findings were all sporadic and transient and none led to aberrant ventricular rhythm. Review of ECG and adverse event data provided no evidence for syncope, seizure, or ventricular tachycardia or fibrillation.
Physiological Effects
Buprenorphine in IV (2, 4, 8, 12 and 16 mg) and sublingual (12 mg) doses have been administered to opioid-experienced subjects who were not physically dependent to examine cardiovascular, respiratory, and subjective effects at doses comparable to those used for treatment of opioid dependence. Compared to placebo, there were no statistically significant differences among any of the treatment conditions for blood pressure, heart rate, respiratory rate, O2 saturation, or skin temperature across time. Systolic BP was higher in the 8 mg group than placebo (3 hour AUC values). Minimum and maximum effects were similar across all treatments. Subjects remained responsive to low voice and responded to computer prompts. Some subjects showed irritability, but no other changes were observed. The respiratory effects of sublingual buprenorphine were compared with the effects of methadone in a double-blind, parallel group, dose ranging comparison of single doses of buprenorphine sublingual solution (1, 2, 4, 8, 16, or 32 mg) and oral methadone (15, 30, 45, or 60 mg) in non-dependent, opioid-experienced volunteers. In this study, hypoventilation not requiring medical intervention was reported more frequently after buprenorphine doses of 4 mg and higher than after methadone. Both drugs decreased O2 saturation to the same degree.
In clinical studies conducted with SUBLOCADE at doses ranging from 50 to 300 mg, no incidences of temperature elevations, or clinically significant lowering of oxygen saturation were observed.
Androgen Deficiency
Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date. Patients presenting with symptoms of androgen deficiency should undergo laboratory evaluation.
12.3. Pharmacokinetics
Absorption
The pharmacokinetics (PK) of buprenorphine following subcutaneous injection of SUBLOCADE was evaluated in subjects with opioid use disorder after single doses (50 mg to 200 mg) and repeated doses (50 to 300 mg) separated by 28 days for up to 12 injections.
After SUBLOCADE injection, an initial buprenorphine peak was observed and the median Tmax occurred at 24 hours after injection. After the initial buprenorphine peak, the plasma buprenorphine concentrations decreased slowly to a plateau. Steady-state was achieved at 4-6 months. Observed mean buprenorphine concentrations levels for Cavg, Cmax and Cmin are presented in Table 6.
Table 6. Comparison of Buprenorphine Mean Pharmacokinetic Parameters Between SUBUTEX and SUBLOCADE:
| Pharmacokinetic parameters | SUBUTEX daily stabilization | SUBLOCADE | |||
| Mean | 12 mg (steady-state) | 24 mg (steady-state) | 300 mg# (1st injection) | 100 mg* (steady-state) | 300 mg* (steady-state) |
| Cavg,ss (ng/mL) | 1.71 | 2.91 | 2.19 | 3.21 | 6.54 |
| Cmax,ss (ng/mL) | 5.35 | 8.27 | 5.37 | 4.88 | 10.12 |
| Cmin,ss (ng/mL) | 0.81 | 1.54 | 1.42† | 2.48 | 5.01 |
# Exposure after 1 injection of 300 mg SUBLOCADE following 24 mg SUBUTEX stabilization
† Mean plasma concentration of 1.86 ng/mL was observed on last day of the dosing interval (Day 29)
* Steady-state exposure after 4 injections of 100 mg or 300 mg SUBLOCADE, following 2 injections of 300 mg SUBLOCADE
Distribution
Buprenorphine is approximately 96% protein bound, primarily to alpha and beta globulin.
Elimination
Buprenorphine is metabolized and eliminated in urine and feces. The apparent terminal plasma half-life of buprenorphine following subcutaneous injection of SUBLOCADE ranged between 43 to 60 days as a result of the slow release of buprenorphine from the subcutaneous depot.
Metabolism
Buprenorphine is metabolized to its major metabolite, norbuprenorphine, primarily by CYP3A4. Norbuprenorphine can further undergo glucuronidation. Norbuprenorphine has been found to bind opioid receptors in vitro; however, it has not been studied clinically for opioid-like activity. Norbuprenorphine steady-state plasma concentrations in humans after subcutaneous injection of SUBLOCADE are low compared to buprenorphine (AUC norbuprenorphine/buprenorphine ratio of 0.20 to 0.40).
Excretion
A mass balance study of buprenorphine administered by IV infusion in humans showed complete recovery of radiolabel in urine (30%) and feces (69%) collected up to 11 days after dosing. Almost all of the dose was accounted for in terms of buprenorphine, norbuprenorphine, and two unidentified buprenorphine metabolites. In urine, most of buprenorphine and norbuprenorphine were conjugated (buprenorphine: 1% free and 9.4% conjugated; norbuprenorphine: 2.7% free and 11% conjugated). In feces, almost all of the buprenorphine and norbuprenorphine were free (buprenorphine: 33% free and 5% conjugated; norbuprenorphine: 21% free and 2% conjugated).
Drug Interaction Studies
CYP3A4 Inhibitors and Inducers
The effects of co-administered CYP3A4 inhibitors and inducers on buprenorphine exposure in subjects treated with SUBLOCADE have not been studied; however, such interactions have been established in studies using transmucosal buprenorphine. The effects of buprenorphine may be dependent on the route of administration.
Buprenorphine is metabolized to norbuprenorphine primarily by cytochrome CYP3A4; therefore, potential interactions may occur when SUBLOCADE is given concurrently with agents that affect CYP3A4 activity. The effects of co-administered CYP3A4 inducers or inhibitors have been established in studies using transmucosal buprenorphine. Patients who transfer to SUBLOCADE treatment from a regimen of transmucosal buprenorphine used concomitantly with CYP3A4 inhibitors (e.g., ketoconazole), macrolide antibiotics (e.g., erythromycin), or HIV protease inhibitors, or CYP3A4 inducer (e.g., phenobarbital, carbamazepine, phenytoin, rifampicin) should be monitored to ensure that the plasma buprenorphine level provided by SUBLOCADE is adequate and not excessive [see Drug Interactions (7)].
Buprenorphine has been found to be a CYP2D6 and CYP3A4 inhibitor and its major metabolite, norbuprenorphine, has been found to be a moderate CYP2D6 inhibitor in in vitro studies employing human liver microsomes. However, the plasma concentrations of buprenorphine and norbuprenorphine resulting from therapeutic SUBLOCADE doses are not expected to significantly affect metabolism of other co-medications.
Specific Populations
Based on population pharmacokinetic analyses, age, sex and race do not have a clinically meaningful effect on PK of SUBLOCADE.
Hepatic Impairment
The effect of hepatic impairment on the pharmacokinetics of SUBLOCADE has not been studied. However, the effect of hepatic impairment on the PK of buprenorphine has been evaluated in a study using 2 mg/0.5 mg buprenorphine/naloxone sublingual tablet in subjects with various degrees of hepatic impairment as indicated by Child-Pugh criteria. While no clinically relevant changes were observed in subjects with mild hepatic impairment, buprenorphine plasma exposure was increased by 64% and 181% in subjects with moderate and severe hepatic impairment, respectively, compared to healthy subjects [see Use in Specific Populations (8.6)].
Renal Impairment
The effect of renal impairment on the pharmacokinetics of SUBLOCADE has not been studied. Clinical studies of SUBLOCADE did not include subjects with severe renal impairment.
Less than 1% is excreted as unchanged buprenorphine in urine following IV buprenorphine administration. No differences in buprenorphine pharmacokinetics were observed between 9 dialysis-dependent and 6 normal patients following IV administration of 0.3 mg buprenorphine [see Use in Specific Populations (8.7)].
Population PK analyses indicated no notable relationship between creatinine clearance and steady-state buprenorphine plasma concentrations.
HCV infection
In subjects with HCV infection but no sign of hepatic impairment, the changes in the mean Cmax, AUC0-last, and half-life values of buprenorphine were not clinically significant in comparison to healthy subjects without HCV infection. No dose adjustment is needed in patients with HCV infection.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
Long-term studies in animals performed to evaluate carcinogenic potential of SUBLOCADE have not been conducted. However, the carcinogenic potential of the active drug substance in SUBLOCADE, buprenorphine, has been evaluated in Sprague-Dawley rats and CD-1 mice.
In the carcinogenicity study conducted in Sprague-Dawley rats, buprenorphine was administered in the diet at doses of 0.6, 5.5, and 56 mg/kg/day (approximately 0.5, 5, and 50 times the recommended human monthly SC dose of 300 mg of buprenorphine) for 27 months. A statistically significant dose-related increase in Leydig cell tumors occurred. In an 86 week study in CD-1 mice, buprenorphine was not carcinogenic at dietary doses up to 100 mg/kg/day (approximately 45 times the recommended human monthly SC dose of 300 mg of buprenorphine).
NMP, an excipient in SUBLOCADE, produced an increase in hepatocellular adenomas and carcinomas in male and female mice at 6 and 8 times the maximum daily dose (MDD) of NMP via SUBLOCADE. The clinical significance of these findings is unclear. No tumors were noted at 1 and 1.3 times the MDD. In 2-year inhalation and dietary studies in rats, NMP did not result in evidence of carcinogenicity.
Mutagenicity
No evidence of mutagenic potential for subcutaneous SUBLOCADE was found in in vivo subcutaneous micronucleus test using rats' marrow.
Mutagenic potential for buprenorphine was studied in a series of tests utilizing gene, chromosome, and DNA interactions in both prokaryotic and eukaryotic systems. Results were negative in yeast (S. cerevisiae) for recombinant, gene convertant, or forward mutations; negative in Bacillus subtilis “rec” assay, negative for clastogenicity in CHO cells, Chinese hamster bone marrow and spermatogonia cells, and negative in the mouse lymphoma L5178Y assay.
Results were equivocal in the Ames test: negative in studies in two laboratories, but positive for frame shift mutation at a high dose (5 mg/plate) in a third study. Results were positive in the Green-Tweets (E. coli) survival test, positive in a DNA synthesis inhibition (DSI) test with testicular tissue from mice, for both in vivo and in vitro incorporation of [3H]thymidine, and positive in unscheduled DNA synthesis (UDS) test using testicular cells from mice.
Impairment of Fertility
In a fertility study in rats, female mating, fertility, and fecundity indices were unaffected by the SC administration of SUBLOCADE up to 900 mg/kg buprenorphine (approximately 38 times the maximum recommended human dose [MRHD] of 300 mg on an AUC basis). However, higher mean post-implantation loss was observed with SUBLOCADE at 900 mg/kg buprenorphine and at an equivalent level of ATRIGEL alone, which correlated with higher mean number of resorptions and reduced mean number of viable fetuses/litter size. Mean gravid uterine weight and mean final body weight were lower with SUBLOCADE at 900 mg/kg buprenorphine and an equivalent level of ATRIGEL alone, and correlated with higher mean number of resorptions and lower fetal body weights. The NOAEL for female fertility was 900 mg/kg and the NOAEL for female-mediated developmental parameters was 600 mg/kg (approximately 25 times the MRHD on an AUC basis).
Male fertility and reproduction indices were lower as evidenced by abnormal sperm parameters (low motility, low mean number of sperm, and higher percentage of abnormal sperm) with SUBLOCADE at 600 mg/kg and with an equivalent level of ATRIGEL. The NOAEL for male fertility parameters, including sperm analysis, and male-mediated developmental parameters was 300 mg/kg (approximately 32 times the MRHD on an AUC basis).
Adverse effects on testes and male fertility were noted in published study in which rats were treated for 10 weeks with daily oral doses of NMP, an excipient in SUBLOCADE at greater than 11.6 times the MDD and resulted in male-mediated adverse effects on offspring (decreased pup weight and survival) at daily doses 3.5 times the MDD of NMP delivered by SUBLOCADE. No adverse effects were noted at oral doses equivalent to the dose of NMP delivered by SUBLOCADE.
14. Clinical Studies
The key studies from the SUBLOCADE clinical development program that support its use in moderate to severe OUD are a Phase 3 double-blind efficacy and safety study (13-0001, NCT02357901) and an opioid blockade study (13-0002, NCT02044094).
14.1 Study 13-0002, NCT02044094
The opioid blockade study evaluated the blockade of subjective opioid effects, PK and safety of SC injections of SUBLOCADE in 39 subjects with OUD (not treatment-seeking).
The peak (Emax) effect of “Drug Liking” Visual Analog Scale (VAS measurement after challenge with IM. injections of 6 mg and 18 mg hydromorphone (HM) was not inferior (i.e., shown to be not substantially more likeable) compared to the Emax of “Drug Liking” VAS, measured after challenge with placebo (at weeks 1 through 4 following the first injection of 300 mg SUBLOCADE). The noninferiority (NI) margin, the largest difference allowed for the 6 or 18 mg HM VAS to exceed the placebo VAS (the maximum VAS recorded following IM injection of 0 mg HM) before being considered significant, was set at 20. Based on comparison to the historical response to opioid agonists in unblocked subjects, a difference of less than 20 points (on a unipolar scale) between the mean maximum response to hydromorphone and the mean maximum placebo response for the same challenge was considered to indicate near-complete blockade.
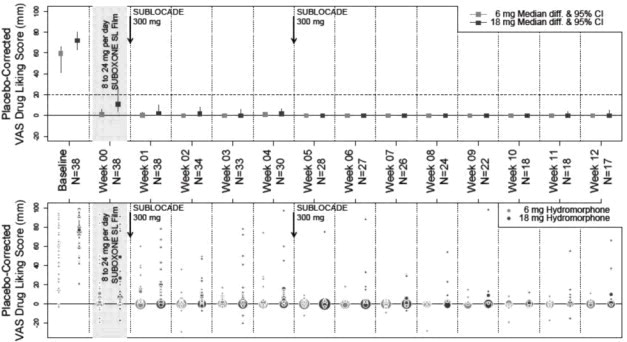
All 12 weeks of the treatment period demonstrated blockade for both 6 mg and 18 mg following SUBLOCADE injections. However, wide variation can be seen in isolated measurements from individual subjects, shown in the figure below. For comparison, stabilization doses of SL buprenorphine in Week 0 failed to provide full blockade to 18 mg of HM. Complete blockade continued throughout the 8 weeks of observation that followed the 2nd SUBLOCADE injection.
Figure 11. Median (95% Confidence Interval) of Placebo-Corrected Drug-Liking Scores by Hydromorphone Dose and by Week:
- Key to Figure: The grey shaded area indicates the period where subjects were stabilized with 8 to 24 mg/day sublingual (SL) buprenorphine; the two vertical arrows represent treatment injections of SUBLOCADE, with 300 mg of buprenorphine.
- The light grey and dark grey squares represent the median Emax drug-liking scores, placebo-corrected (VAS drug liking for that week’s 0 mg dose subtracted) during the hydromorphone challenge of 6 and 18 mg, respectively. This median Placebo-Corrected Emax is shown by treatment week, together with its 95% confidence interval (CI; vertical line). In some cases, 95% CI are not visible as the median was equal to the confidence limit. The horizontal line at 20 mm delineates the non-inferiority margin for opioid blockade. Next to median estimates, individual data are summarized by circles, the area of which is proportional to the number of subjects at that location.
- The X axis shows how many weeks following injection #1 that each weeks' Placebo-Corrected Drug-Liking Score was measured. Beneath that treatment week indicator, is the number of subjects (N) who provided those VAS measurements for all three challenges with placebo, 6 and 18 mg hydromorphone.
14.2 Study 13-0001, NCT02357901
The efficacy of SUBLOCADE for the treatment of opioid use disorder was evaluated in a Phase 3, 24- week, randomized, double-blind, placebo-controlled, multicenter trial in treatment-seeking patients who met the DSM-5 criteria for moderate or severe opioid use disorder. Patients were randomized to one of following dosing regimens: 6 once-monthly 300 mg doses, 2 once-monthly 300 mg doses followed by 4 once-monthly 100 mg doses, or 6 once-monthly SC injections of placebo. All doses were administered by a physician or suitably qualified designee and were separated by 28 ± 2 days. In addition to study medication, all subjects received manual-guided psychosocial support at least once a week (Individual Drug Counseling = IDC).
Prior to the first dose, treatment was initiated with SUBOXONE (buprenorphine/naloxone) sublingual film (SUBOXONE SL Film); doses were adjusted from 8/2mg to 24/6 mg per day over a period of 7-14 days. Patients were randomized to SUBLOCADE injection or placebo after cravings and withdrawal symptoms were clinically controlled. After randomization, supplemental dosing with SUBOXONE SL Film was not permitted during the study.
Efficacy was evaluated over Weeks 5 through 24 based on weekly urine drug screens combined with self-reported use of illicit opioid use. A “grace period” was applied for Weeks 1 through 4 to allow patients to stabilize in treatment. During this period, opioid use, if it occurred, was not considered in the analysis. Missing urine drug screen samples and/or self-reports during Weeks 5-24 were counted as positive for illicit opioids.
A total of 504 patients were randomized 4:4:1:1 [203 subjects in the 300 mg/100 mg group, 201 patients in the 300 mg/300 mg group and 100 patients in the placebo group (2 groups of volume-matched placebo)]. Patients demographics and baseline characteristics are provided in Table 7.
Table 7. Patient Demographics and Baseline Characteristics:
| SUBLOCADE 300/100 mg % | SUBLOCADE 300/300 mg % | Placebo % | |
|---|---|---|---|
| Mean Age (years) | 40.4 | 39.3 | 39.2 |
| Sex | |||
| Male | 66.0 | 67.3 | 64.6 |
| Female | 34.0 | 32.7 | 35.4 |
| Race or Ethnicity | |||
| White | 68.0 | 71.4 | 77.8 |
| Black or African American | 28.9 | 27.6 | 20.2 |
| Hispanic or Latino | 6.2 | 9.2 | 10.1 |
| Substance Use At Screening | |||
| Opioid Use – Injectable Route | 43.3 | 40.8 | 50.5 |
| Tobacco | 91.8 | 92.3 | 92.9 |
| Alcohol | 78.4 | 79.1 | 80.8 |
| Drug Use History | |||
| Cannabinoids | 54.6 | 47.4 | 52.5 |
| Cocaine | 47.4 | 39.8 | 42.4 |
| Amphetamine/Methamphetamine | 25.3 | 14.8 | 19.2 |
| Medical History | |||
| Depression | 14.4 | 11.2 | 13.1 |
| Anxiety | 9.3 | 9.7 | 10.1 |
| Back Pain | 14.9 | 16.3 | 13.1 |
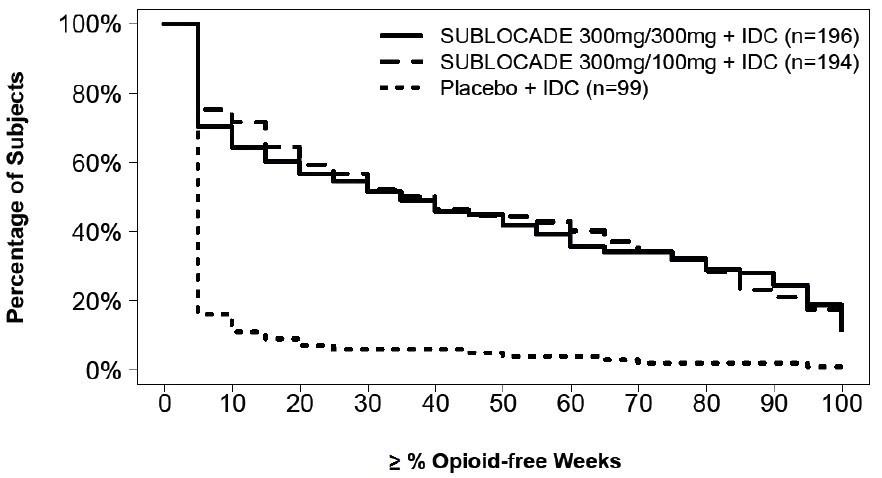
Based on the cumulative distribution function (CDF) of the percentage of urine samples negative for illicit opioids combined with self-reports negative for illicit opioid use collected from Week 5 through Week 24 (Table 8), regardless of dose, SUBLOCADE was superior to the placebo group with statistical significance. The proportion of patients achieving treatment success (defined as patients with ≥80% opioid-free weeks) was statistically significantly higher in both groups receiving SUBLOCADE compared to the placebo group (28.4% [300 mg/100 mg], 29.1% [300 mg/300mg], 2% [placebo]).
For various percentages of opioid-free weeks, Table 8 shows the fraction of patients achieving that criterion. The table is cumulative, so that a patient whose percent of opioid-free weeks is, for example, 50%, is also included at every level of opioid-free week percentage below 50%. Missing values and values after premature discontinuation were considered positive.
Figure 12. Subjects Achieving Varying Percentages of Opioid-Free Weeks:
Table 8. Cumulative Distribution Function of Percentage of Opioid-Free Weeks:
| Number (%) of Subjects | |||
|---|---|---|---|
| SUBLOCADE | SUBLOCADE | ||
| 300mg/100mg + IDC | 300mg/300mg + IDC | Placebo + IDC | |
| Percentage Opioid-Free Weeks | (N=194) | (N=196) | (N=99) |
| ≥0% | 194 (100.0) | 196 (100.0) | 99 (100.0) |
| ≥10% | 139 (71.6) | 126 (64.3) | 11 (11.1) |
| ≥20% | 115 (59.3) | 111 (56.6) | 7 (7.1) |
| ≥30% | 101 (52.1) | 101 (51.5) | 6 (6.1) |
| ≥40% | 90 (46.4) | 90 (45.9) | 6 (6.1) |
| ≥50% | 86 (44.3) | 82 (41.8) | 4 (4.0) |
| ≥60% | 78 (40.2) | 70 (35.7) | 4 (4.0) |
| ≥70% | 66 (34.0) | 67 (34.2) | 2 (2.0) |
| ≥80% | 55 (28.4) | 57 (29.1) | 2 (2.0) |
| ≥90% | 41 (21.1) | 48 (24.5) | 2 (2.0) |
| =100% | 25 (13) | 23 (12) | 1 (1.0) |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.