SUBLOCADE Solution for injection Ref.[27427] Active ingredients: Buprenorphine
Source: FDA, National Drug Code (US) Revision Year: 2021
1. Indications and Usage
SUBLOCADE is indicated for the treatment of moderate to severe opioid use disorder in patients who have initiated treatment with a transmucosal buprenorphine-containing product, followed by dose adjustment for a minimum of 7 days.
SUBLOCADE should be used as part of a complete treatment plan that includes counseling and psychosocial support.
2. Dosage and Administration
2.1 Drug Addiction Treatment Act
Under the Drug Addiction Treatment Act (DATA) codified at 21 U.S.C. 823(g), prescription use of this product in the treatment of opioid dependence is limited to healthcare providers who meet certain qualifying requirements, and who have notified the Secretary of Health and Human Services (HHS) of their intent to prescribe this product for the treatment of opioid dependence and have been assigned a unique identification number that must be included on every prescription.
2.2 Important Dosing and Administration Information
FOR ABDOMINAL SUBCUTANEOUS INJECTION ONLY. DO NOT ADMINISTER SUBLOCADE INTRAVENOUSLY, INTRAMUSCULARLY OR INTRADERMALLY [see Dosage and Administration (2.7), Warnings and Precautions (5.1,5.6)].
- Only healthcare providers should prepare and administer SUBLOCADE.
- Administer SUBLOCADE monthly with a minimum of 26 days between doses.
- Initiating treatment with SUBLOCADE as the first buprenorphine product has not been studied. Initiate SUBLOCADE treatment only following induction and dose-adjustment with a transmucosal buprenorphine-containing product [see Dosage and Administration (2.5)].
- Administer each injection only using the syringe and safety needle included with the product [see Dosage and Administration (2.7)].
2.3 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated for opioid use disorder have the potential for relapse, putting them at risk for opioid overdose, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with SUBLOCADE. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Warnings and Precautions (5.4)].
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with buprenorphine itself. Higher than normal doses and repeated administration of naloxone may be necessary due to the long duration of action of buprenorphine and its affinity for the mu-opioid receptor [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Patient Counseling Information (17)].
2.4 Recommended Dosing
The recommended dose of SUBLOCADE following induction and dose adjustment with transmucosal buprenorphine is 300 mg monthly for the first two months followed by a maintenance dose of 100 mg monthly.
The maintenance dose may be increased to 300 mg monthly for patients who tolerate the 100 mg dose, but do not demonstrate a satisfactory clinical response, as evidenced by self-reported illicit opioid use or urine drug screens positive for illicit opioid use.
A patient who misses a dose should receive the next dose as soon as possible, with the following dose given no less than 26 days later. Occasional delays in dosing up to 2 weeks are not expected to have a clinically significant impact on treatment effect.
For patients in established treatment of 100 mg monthly, there may be instances when allowance for a two-month dosing interval may be appropriate (e.g., extended travel); in those instances, a single 300 mg dose may be given to cover a two-month period. Thereafter, the 100 mg monthly regimen would resume. Patients should be cautioned that the peak plasma level after injection of a 300 mg dose will be higher than their usual monthly dose and that they may experience sedation or other buprenorphine-related effects.
2.5 Patient Selection
Patients appropriate for SUBLOCADE are adults who have initiated treatment on a transmucosal buprenorphine-containing product delivering the equivalent of 8 to 24 mg of buprenorphine daily. The patient may only be transitioned to SUBLOCADE after a minimum of 7 days.
Initiation of treatment with transmucosal buprenorphine-containing products should be based on instructions in their appropriate product label. One SUBOXONE (buprenorphine and naloxone) 8 mg/2 mg sublingual tablet provides equivalent buprenorphine exposure to one SUBUTEX (buprenorphine HCl) 8 mg sublingual tablet or one Bunavail (buprenorphine and naloxone) 4.2 mg/0.7 mg buccal film or one Zubsolv (buprenorphine and naloxone) 5.7 mg/1.4 mg sublingual tablet.
2.6 Clinical Supervision
Periodic assessment is necessary to determine effectiveness of the treatment plan and overall patient progress. When evaluating the patient, examine the injection site for signs of infection or evidence of tampering or attempts to remove the depot.
Due to the chronic nature of opioid use disorder, the need for continuing medication-assisted treatment should be re-evaluated periodically. There is no maximum recommended duration of maintenance treatment. For some patients, treatment may continue indefinitely. If considering stopping treatment, the clinical status of the patient should be considered.
If SUBLOCADE is discontinued, its extended-release characteristics should be considered and the patient should be monitored for several months for signs and symptoms of withdrawal and treated appropriately. After steady-state has been achieved (4-6 months), patients discontinuing SUBLOCADE may have detectable plasma levels of buprenorphine for twelve months or longer. The correlation between plasma concentrations of buprenorphine and those detectable in urine is not known.
2.7 Instructions for Use
IMPORTANT INFORMATION:
- For abdominal subcutaneous injection only. Do not inject intravenously, intramuscularly, or intradermally [see Warnings and Precautions (5.1, 5.6)].
- To be prepared and administered by a healthcare provider only.
- Please read the instructions carefully before handling the product.
- As a universal precaution, always wear gloves.
- Remove SUBLOCADE from the refrigerator prior to administration. The product requires at least 15 minutes to reach room temperature. Do not open the foil pouch until the patient has arrived for his or her injection.
- Discard SUBLOCADE if left at room temperature for longer than 7 days.
- Do not attach the needle until time of administration.
STEP 1: GETTING READY
Remove the foil pouch and safety needle from the carton. Open the pouch and remove the syringe.
Discard the oxygen absorber pack. It is not needed.
Figure 1:
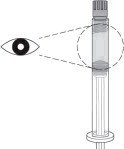
STEP 2: CHECK THE LIQUID CLARITY
Check that the medication does not contain contaminants or particles. SUBLOCADE ranges from colorless to yellow to amber. Variations of color within this range do not affect the potency of the product.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Figure 2:
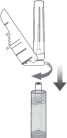
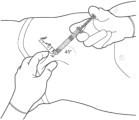
STEP 3: ATTACH THE SAFETY NEEDLE
Remove the cap from the syringe and the safety needle supplied in the carton from its sterile package.
Gently twist the needle clockwise until it is tight and firmly attached.
Do not remove the plastic cover from the needle.
Figure 3:
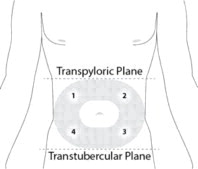
STEP 4: PREPARE THE ABDOMINAL INJECTION SITE
Choose an injection site on the abdomen between the transpyloric and transtubercular planes with adequate subcutaneous tissue that is free of skin conditions (e.g., nodules, lesions, excessive pigment). It is recommended that the patient is in the supine position.
Do not inject into an area where the skin is irritated, reddened, bruised, infected or scarred in any way.
Clean the injection site well with an alcohol swab.
To avoid irritation, rotate injection sites following a pattern similar to the illustration in Figure 4. Record the location of the injection to ensure that a different site is used at the time of the next injection.
Figure 4:
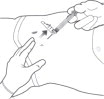
STEP 5: REMOVE EXCESS AIR FROM SYRINGE
Hold the syringe upright for several seconds to allow air bubbles to rise. Due to the viscous nature of the medication, bubbles will not rise as quickly as those in an aqueous solution.
Remove needle cover and slowly depress the plunger to push out the excess air from the syringe.
- Small bubbles may remain in the medication. Large air gaps, however, can be minimized by pulling back on the plunger rod to pop air bubbles prior to expelling the air very slowly. Air should be expelled very carefully to avoid loss of medication.
If medication is seen at the needle tip, pull back slightly on the plunger to prevent medication spillage.
Figure 5:
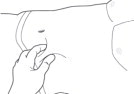
STEP 6: PINCH THE INJECTION SITE
Pinch the skin around the injection area. Be sure to pinch enough skin to accommodate the size of the needle. Lift the adipose tissue from the underlying muscle to prevent accidental intramuscular injection.
Figure 6:
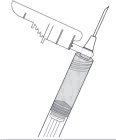
STEP 7: INJECT THE MEDICATION
SUBLOCADE is for subcutaneous injection only. Do not inject intravenously, intramuscularly, or intradermally [see Warnings and Precautions (5.1, 5.6)].
Insert needle fully into the abdominal subcutaneous tissue. Actual angle of injection will depend on the amount of subcutaneous tissue.
Use a slow, steady push to inject the medication. Continue pushing until all of the medication is given.
Figure 7:
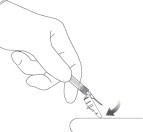
STEP 8: WITHDRAW THE NEEDLE
Withdraw the needle at the same angle used for insertion and release the pinched skin.
Do not rub the injection area after the injection. There may be a small amount of blood or fluid at the injection site; wipe with a cotton ball or gauze before applying a gauze pad or bandage using minimal pressure.
Figure 8:
STEP 9: LOCK THE NEEDLE GUARD AND DISCARD THE SYRINGE
Lock the needle guard into place by pushing it against a hard surface such as a table (Figure 9).
Dispose of all syringe components in a secure sharps disposal container.
Figure 9:
STEP 10: INSTRUCT THE PATIENT
Advise the patient that they may have a lump for several weeks that will decrease in size over time. Instruct the patient not to rub or massage the injection site and to be aware of the placement of any belts or clothing waistbands.
2.8 Limits on Distribution
SUBLOCADE is subject to a risk evaluation and mitigation strategy (REMS) program that includes, among other elements, a restricted distribution system. The purpose of the restricted distribution system is to ensure that SUBLOCADE is only administered by a health care provider [see Warnings and Precautions (5.2)].
2.9 Removal of the Depot
In the event the depot must be removed, it can be surgically excised under local anesthesia within 14 days of injection. Only the most recently-injected depot can be removed.
The removed depot should be handled with adequate security, accountability, and proper disposal, per facility procedure for a Schedule III drug product and pharmaceutical biohazardous waste, and per applicable federal, state, and local regulations.
The residual plasma concentrations from previous injections will decrease gradually over subsequent months [see Clinical Pharmacology (12.3)].
Patients who have the depot removed should be monitored for signs and symptoms of withdrawal and treated appropriately [see Warnings and Precautions (5.9)].
10. Overdosage
Clinical Presentation
The manifestations of acute overdose include pinpoint pupils, sedation, hypotension, respiratory depression, and death.
Treatment of Overdose
In the event of overdose, the respiratory and cardiac status of the patient should be monitored carefully. When respiratory or cardiac functions are depressed, primary attention should be given to the re-establishment of adequate respiratory exchange through provision of a patent airway and institution of assisted or controlled ventilation. Oxygen, IV fluids, vasopressors, and other supportive measures should be considered as indicated. Naloxone may be of value for the management of buprenorphine overdose. Higher than normal doses and repeated administration may be necessary.
Clinicians should consider the potential role and contribution of buprenorphine, other opioids, and other CNS depressant drugs in a patient’s clinical presentation. Clinical data are limited with regards to the possible surgical removal of the depot. Two cases of surgical removal were reported in premarketing clinical studies.
16.2. Storage and Handling
Store refrigerated at 2-8°C (35.6-46.4°F).
Once outside the refrigerator this product may be stored in its original packaging at room temperature, 15–30°C (59–86°F), for up to 7 days prior to administration. Discard SUBLOCADE if left at room temperature for longer than 7 days.
SUBLOCADE is a Schedule III drug product. Handle with adequate security and accountability. After administration, syringes should be properly disposed, per facility procedure for a Schedule III drug product, and per applicable federal, state, and local regulations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.