TAMOLTRA Film coated tablet Ref.[115184] Active ingredients: Paracetamol Tramadol
Source: Health Products Regulatory Authority (ZA) Revision Year: 2025 Publisher: Pharma Dynamics (Pty) Ltd, 1<sup>st</sup> Floor Grapevine House, Steenberg Office Park, Silverwood Close, Westlake, Cape Town, 7945, South Africa, Tel: 0860-PHARMA (742 762) / +2721 707 7000
Therapeutic indications
TAMOLTRA and TAMOLTRA FORTE are indicated for the management of moderate to moderately severe pain in adults.
TAMOLTRA and TAMOLTRA FORTE are not recommended for minor pain that may be treated adequately through lesser means.
Posology and method of administration
Posology
TAMOLTRA: To be used in adults and children over 16 years of age.
TAMOLTRA FORTE: To be used in adults.
DO NOT EXCEED THE RECOMMENDED DOSE.
TAMOLTRA: Adults and children over the age of 16
For the management of pain, the recommended dose of TAMOLTRA is 1 or 2 tablets every 4 to 6 hours, as needed for pain relief, up to a maximum of 8 tablets per day.
TAMOLTRA FORTE: Adults
The recommended dose of TAMOLTRA FORTE is 1 tablet every 4 to 6 hours as needed. The maximum total dose per day is 4 tablets.
As with all analgesic medicines, a titration period of several days with gradual dose increases at the initiation of TAMOLTRA and TAMOLTRA FORTE therapy may be beneficial for some patients.
Clinical studies with tramadol in patients with moderate to moderately severe chronic pain indicated that the tolerability of tramadol can be improved by starting at a lower dose with gradual upward titration to reach doses that provide sufficient pain relief.
Special populations
Elderly population (65 years of age and older)
No overall differences, about safety or pharmacokinetics, were noted between subjects ≥65 years of age and younger subjects.
Renal insufficiency/dialysis
In patients with renal insufficiency, the elimination of tramadol is delayed. In these patients, prolongation of the dosage intervals should be carefully considered according to the patients' requirements.
For patients with creatinine clearance <30 mL/min, the dosing interval should be increased but should not exceed 2 tablets every 12 hours.
Hepatic impairment
TAMOLTRA and TAMOLTRA FORTE should not be used in patients with moderate to severe liver impairment (see section 4.3).
Paediatric population
TAMOLTRA: Not indicated for use in children under the age of 16 years, as safety and efficacy have not been established.
TAMOLTRA FORTE: Not recommended in patients under 18 years old.
Method of administration
For oral use.
The coated tablets must be swallowed whole, with a sufficient quantity of liquid. They must not be broken or chewed.
Tablets can be administered without regard to food.
Missed dose
Doctors should advise patients who forget to take TAMOLTRA or TAMOLTRA FORTE to take a dose as soon as possible and then continue with the normal dose. Patients should not take a double dose to compensate for the missed dose.
Overdose
Signs and symptoms
The clinical presentation of overdosage may include the signs and symptoms of tramadol toxicity, paracetamol toxicity or both.
Tramadol
The initial symptoms of tramadol overdosage may include respiratory depression and/or seizures.
Paracetamol
Symptoms of paracetamol overdosage in the first 24 hours include pallor, nausea, vomiting, anorexia, and possibly abdominal pain. Mild symptoms during the first two days of acute poisoning do not reflect the potential seriousness of the overdosage.
Liver damage may become apparent 12 to 48 hours or later after ingestion, initially by elevation of the serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentration and prolongation of prothrombin time. Liver damage may lead to encephalopathy, coma, and death. Acute renal failure with acute tubular necrosis may develop even in the absence of severe liver damage.
Abnormalities of glucose metabolism and metabolic acidosis may occur. Cardiac dysrhythmias have been reported.
Management of overdose
Tramadol
Primary attention should be given to maintaining adequate ventilation along with general supportive treatment. While naloxone will reverse some, but not all symptoms caused by overdosage, the risk of seizures is also increased with naloxone administration. Treatment of restlessness and/or convulsions is symptomatic and supportive (benzodiazepines/barbiturates).
Tramadol is minimally eliminated from the serum by haemodialysis or haemofiltration. Treatment of acute intoxication with TAMOLTRA and TAMOLTRA FORTE with haemodialysis or haemofiltration alone is therefore not suitable for detoxification.
Paracetamol
Prompt treatment is essential. In the event of an overdosage, consult a medical practitioner immediately, or take the person to a hospital directly. A delay in starting treatment may mean that antidote is given too late to be effective. Evidence of liver damage is often delayed until after the time for effective treatment has lapsed. Susceptibility to paracetamol toxicity is increased in patients who have taken repeated high doses (greater than 5-10 g/day) of paracetamol for several days, in chronic alcoholism, chronic liver disease, AIDS, malnutrition, and with the use of drugs that induce liver microsomal oxidation such as barbiturates, isoniazid, rifampicin, phenytoin and carbamazepine.
Treatment for paracetamol overdosage
Although evidence is limited it is recommended that any adult person who have ingested 5-10 g or more of paracetamol (or child who has had more than 140 mg/kg) within the preceding 4 hours should have the stomach emptied by gastric lavage (emesis may be adequate for children) and a single dose of 50 g activated charcoal given via the lavage tube. Ingestion of amounts of paracetamol smaller than this requires treatment in patients susceptible to paracetamol poisoning. In patients who are stuperose or comatose, endotracheal tubing should precede gastric lavage in order to avoid aspiration.
N-acetylcysteine should be administered to all cases of suspected overdose as soon as possible, preferably within eight hours of overdosage, Treatment up to 36 hours after ingestion may still be of benefit, especially if more than 150 mg/kg of paracetamol was taken. An initial dose of 150 mg/kg N-acetylcysteine in 200 mL dextrose injection given intravenously over 15 minutes, followed by an infusion of 50 mg/kg in 500 mL dextrose injection over the next four hours, and then 100 mg/kg in 1000 mL dextrose injection over the next sixteen hours. The volume of intravenous fluid should be modified for children.
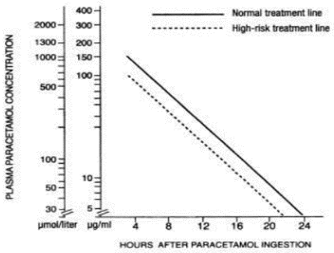
Although the oral formulation is not the treatment of choice, 140 mg/kg dissolved in water may be administered initially, followed by 70 mg/kg every four hours for seventeen doses. A plasma paracetamol level should be determined four hours after ingestion in all cases of suspected overdosage. Levels done before four hours, unless high, may be misleading. Patients at risk of liver damage, and hence requiring continued treatment with N-acetylcysteine, can be identified according to their plasma paracetamol overdose nomogram.
Those whose plasma paracetamol levels are above the “normal treatment line”, should continue N-acetylcysteine treatment with 100 mg/kg IV over sixteen hours repeatedly until recovery. Patients with increased susceptibility to liver damage as identified above, should continue treatment if concentrations are above the “high risk treatment line”. Prothrombin index correlates best with survival.
Monitor all patients with significant ingestions for at least ninety-six hours. Further treatment is symptomatic and supportive.
Shelf life
3 years.
Special precautions for storage
TAMOLTRA: Store at or below 30°C in a cool, dry place.
TAMOLTRA FORTE: Store at or below 25°C in a cool, dry place.
Keep the blisters in the carton until required for use.
Nature and contents of container
TAMOLTRA is available in blister packs consisting of PVC/PVDC, white film and aluminium foil, with 20, 30 or 60 tablets packed in an outer carton.
TAMOLTRA FORTE is available in blister packs consisting of PVC/PVDC, white film and aluminium foil, with 10, 20, 30, 40, 50, 60, 70, 80, 90 or 100 tablets packed in an outer carton.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.