TEKTURNA Film-coated tablet Ref.[50359] Active ingredients: Aliskiren
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
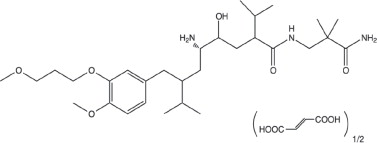
Tekturna contains aliskiren hemifumarate, adirect renin inhibitor. Aliskiren hemifumarate is chemically described as (2S,4S,5S,7S)N(2-carbamoyl-2-methylpropyl)-5-amino-4-hydroxy- 2,7-diisopropyl-8-[4-methoxy-3-(3-methoxypropoxy)phenyl]-octanamide hemifumarate and its structural formula is:
Molecular formula: C30H53N3O6 • 0.5 C4H4O4
Aliskiren hemifumarate is a white to slightly yellowish crystalline powder with a molecular weight of 609.8 (free base – 551.8). It is soluble in phosphate buffer, n-octanol, and highly soluble in water.
Tekturna is available as film-coated tablets, which contains 165.75 mg or 331.5 mg aliskiren hemifumerate (equivalent to 150 mg or 300 mg aliskiren) and the following excipients: crospovidone; magnesium stearate; microcrystalline cellulose; povidone; silica, colloidal anhydrous; hypromellose; macrogol; talc; iron oxide, black (E172); iron oxide, red (E172); titanium dioxide (E171).
| Dosage Forms and Strengths |
|---|
|
150 mg light pink biconvex round tablet, imprinted NVR/IL (Side 1/Side 2). 300 mg light red biconvex ovaloid round tablet, imprinted NVR/IU (Side 1/Side 2). |
| How Supplied | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Tekturna tablets are supplied as a light-pink, biconvex round tablet containing 150 mg of aliskiren, and as a light-red biconvex ovaloid tablet containing 300 mg of aliskiren. Tablets are imprinted with NVR on one side and IL, IU, on the other side of the 150 mg and 300 mg tablets, respectively. All strengths are packaged in bottles and unit-dose blister packages (10 strips of 10 tablets) as described below in Table 7. Table 7. Tekturna Tablets Supply:
|
||||||||||||||||||||||||||||
Drugs
| Drug | Countries | |
|---|---|---|
| TEKTURNA | United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.