TYLENOL Tablets, Capsule, Suspension, Powder Ref.[50413] Active ingredients: Paracetamol
Source: Health Products and Food Branch (CA) Revision Year: 2021
Action and clinical pharmacology
Mechanism of Action
Acetaminophen is a centrally acting analgesic and antipyretic drug. Although the precise mechanism of action is not totally understood, work by Boutaud86 suggests acetaminophen is an inhibitor of the peroxidase portion of cyclooxygenase (prostaglandin H synthase inhibitor). Depending on the redox state and substrate concentrations surrounding the enzymes, acetaminophen may or may not have a significant inhibitory effect. This accounts for its selective activity on pain and fever with little anti-inflammatory effect87.
It is postulated that the analgesic effect is produced by elevation of the pain threshold and the antipyretic effect is produced through action on the hypothalamic heat-regulating centre.
The optimal effective analgesic dose of acetaminophen was demonstrated in dental pain studies and is 1000 mg every four to six hours, up to 4000 mg daily. At least 500 published and unpublished controlled clinical trials in adults and children have evaluated acetaminophen for the relief of pain or fever. These studies include single and multiple dose treatments. Most studies were less than 14 days in duration, although the longest study duration was two years. No significant safety issues were reported in any of these studies.
Moreover, at recommended doses, acetaminophen has not been shown to increase the risk of developing renal diseases21,35,88,89 or upper gastrointestinal ulceration/bleeding22,23,90-92. This observation is consistent with its minimal inhibitory effect on peripheral prostaglandin synthesis93 and on gastric prostaglandin synthesis94.
Acetaminophen is considered equipotent to ASA and ibuprofen, within the recommended OTC dosing ranges, in its analgesic and antipyretic effects. Acetaminophen at recommended doses does not cause the type of gastrointestinal complications associated with NSAID-containing products, such as gastric irritation, gastric erosions, occult or overt gastrointestinal blood loss, or ulcers. Unlike these drugs, however, it has no antiinflammatory effect at clinically relevant doses in humans.
Pharmacokinetics
A. Absorption
Oral acetaminophen is rapidly and almost completely absorbed from the gastrointestinal tract primarily in the small intestine. This absorption process occurs by passive transport. Peak plasma concentrations occur within 0.4 to 1 hour depending on the product formulation. Although high-fat foods delay the time to peak concentration for up to an hour, the dose is completely absorbed.
B. Distribution
Acetaminophen is uniformly distributed throughout most body fluids, but not in fatty tissue. As a result, the volume of distribution in adults ranges between 0.8 and 1.0 L/kg32,95. Since acetaminophen has low protein binding in plasma of only 10% to 25%, it does not compete with drugs that are highly protein bound96,97.
C. Metabolism
Acetaminophen is primarily metabolized by the liver via three principal separate pathways68:
a) Conjugation with glucuronide
b) Conjugation with sulfate
c) Oxidation via the cytochrome P450 mixed function oxidase system.
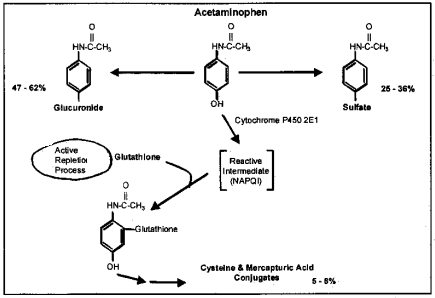
Both the glucuronic and oxidative pathways adhere to a first-order rate process, which means the concentration of acetaminophen metabolized increases as the concentration in the liver increases. The sulfate pathway adheres to Michaelis-Menten kinetics, which means the concentration of acetaminophen metabolized remains constant once the concentration in the liver increases above a saturation level. A schematic of acetaminophen metabolism is shown in Figure 2.
The major metabolic pathway is glucuronidation, where 47% to 62% of the acetaminophen dose conjugates with glucuronide. These glucuronide conjugates are inactive and nontoxic, and are secreted in bile and eliminated in the urine.
The second major pathway is sulfation, where 25% to 36% of the dose conjugates with sulfate. These sulfate ester conjugates are also inactive and nontoxic and are excreted in the urine.
The third pathway is oxidation, where 5% to 8% of the dose is metabolized via the cytochrome P450 enzyme system. The cytochrome P450 isoenzyme that is primarily responsible is CYP2E1. When acetaminophen is metabolized by CYP2E1, it forms a highly reactive intermediate, N-acetyl-p-benzoquinoneimine (NAPQI). Since NAPQI is highly reactive, it cannot be measured outside the liver nor can it accumulate. This intermediate is rapidly inactivated by hepatocellular stores of glutathione to form cysteine and mercapturate conjugates, which are both inactive and nontoxic. These conjugates are excreted in the urine.
Figure 2. Acetaminophen Metabolism:
D. Elimination
Acetaminophen undergoes first-order elimination from the body, and has a short plasma half-life that ranges from 2 to 3 hours in healthy young and elderly adults13,98-104 and from 1.5 to 2.9 hours in children105-109. Since acetaminophen clears rapidly from the body, repeated doses do not lead to accumulation of acetaminophen plasma concentrations.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.