VIMOVO Delayed release tablet Ref.[50562] Active ingredients: Esomeprazole Naproxen
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
The active ingredients of VIMOVO are naproxen which is an NSAID and esomeprazole magnesium which is a Proton Pump Inhibitor (PPI).
VIMOVO (naproxen and esomeprazole magnesium) is combination of a nonsteroidal anti- inflammatory drug and a PPI available as an oval, yellow, multi-layer, delayed-release tablet combining an enteric-coated naproxen core and an immediate-release esomeprazole magnesium layer surrounding the core.
Each strength contains either 375 mg of naproxen and 20 mg of esomeprazole (equivalent to 22.3 mg esomeprazole magnesium trihydrate) or 500 mg of naproxen and 20 mg of esomeprazole (equivalent to 22.3 mg esomeprazole magnesium trihydrate) for oral administration. The inactive ingredients are carnauba wax, colloidal silicon dioxide, croscarmellose sodium, iron oxide yellow, glyceryl monostearate, hypromellose, iron oxide black, magnesium stearate, methacrylic acid copolymer dispersion, methylparaben, polysorbate 80, polydextrose, polyethylene glycol, povidone, propylene glycol, propylparaben, titanium dioxide, and triethyl citrate.
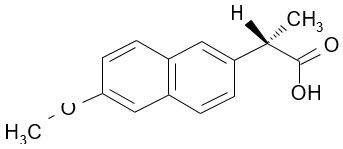
The chemical name for naproxen is (S)-6-methoxy-α-methyl-2-naphthaleneacetic acid.
Naproxen has the following structure:
Naproxen has a molecular weight of 230.26 and a molecular formula of C14H14O3.
Naproxen is an odorless, white to off-white crystalline substance. It is lipid soluble, practically insoluble in water at low pH and freely soluble in water at high pH. The octanol/water partition coefficient of naproxen at pH 7.4 is 1.6 to 1.8.
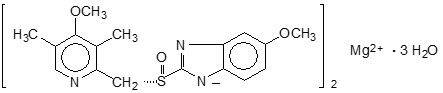
The chemical name for esomeprazole is bis(5-methoxy-2-[(S)-[(4-methoxy-3,5-dimethyl-2-pyridinyl)methyl]sulfinyl]-1Hbenzimidazole-1-yl) magnesium trihydrate. Esomeprazole is the S isomer of omeprazole, which is a mixture of the S- and R- isomers. Its molecular formula is (C17H18N3O3S)2Mg × 3 H2O with molecular weight of 767.2 as a trihydrate and 713.1 on an anhydrous basis.
The structural formula is:
The magnesium salt is a white to slightly colored crystalline powder. It contains 3 moles of water of solvation and is slightly soluble in water.
The stability of esomeprazole magnesium is a function of pH; it rapidly degrades in acidic media, but it has acceptable stability under alkaline conditions. At pH 6.8 (buffer), the half-life of the magnesium salt is about 19 hours at 25°C and about 8 hours at 37°C.
| Dosage Forms and Strengths |
|---|
|
VIMOVO is an oval, yellow, delayed-release tablets for oral administration containing either:
|
| How Supplied | ||||
|---|---|---|---|---|
|
VIMOVO (375 mg naproxen/20 mg esomeprazole magnesium) delayed-release tablets are oval, yellow film-coated tablets printed with 375/20 in black ink, supplied as:
VIMOVO (500 mg naproxen/20 mg esomeprazole magnesium) delayed-release tablets are oval, yellow film-coated tablets printed with 500/20 in black ink, supplied as:
Distributed by: Horizon Medicines, LLC., Deerfield, IL 60015 |
Drugs
| Drug | Countries | |
|---|---|---|
| VIMOVO | Austria, Brazil, Canada, Estonia, Spain, Finland, Ireland, Lithuania, Netherlands, Romania, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.