ZESTORETIC Tablet Ref.[10627] Active ingredients: Hydrochlorothiazide Lisinopril
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
ZESTORETIC (Lisinopril and Hydrochlorothiazide) combines an angiotensin converting enzyme inhibitor, lisinopril, and a diuretic, hydrochlorothiazide.
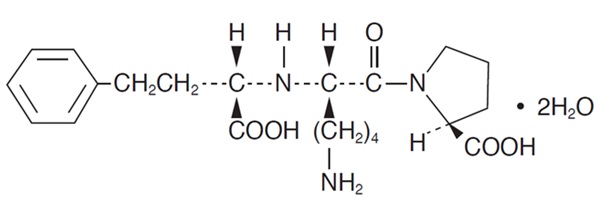
Lisinopril, a synthetic peptide derivative, is an oral long-acting angiotensin converting enzyme inhibitor. It is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5 . 2H2O and its structural formula is:
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.53. It is soluble in water, sparingly soluble in methanol, and practically insoluble in ethanol.
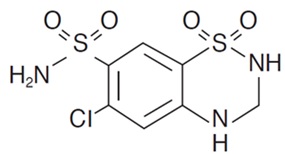
Hydrochlorothiazide is 6-chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its empirical formula is C7H8ClN3O4S2 and its structural formula is:
Hydrochlorothiazide is a white, or practically white, crystalline powder with a molecular weight of 297.72, which is slightly soluble in water, but freely soluble in sodium hydroxide solution.
ZESTORETIC is available for oral use in three tablet combinations of lisinopril with hydrochlorothiazide: ZESTORETIC 10-12.5 containing 10 mg lisinopril and 12.5 mg hydrochlorothiazide; ZESTORETIC 20-12.5 containing 20 mg lisinopril and 12.5 mg hydrochlorothiazide; and, ZESTORETIC 20-25 containing 20 mg lisinopril and 25 mg hydrochlorothiazide.
Inactive Ingredients:
10-12.5 Tablets - calcium phosphate, magnesium stearate, mannitol, red ferric oxide, corn starch, yellow ferric oxide.
20-12.5 Tablets - calcium phosphate, magnesium stearate, mannitol, corn starch.
20-25 Tablets - calcium phosphate, magnesium stearate, mannitol, red ferric oxide, corn starch, yellow ferric oxide.
| How Supplied |
|---|
|
ZESTORETIC 10-12.5 Tablets: Peach, round, biconvex, uncoated tablets identified with "141" debossed on one side and "ZESTORETIC" on the other side are supplied in bottles of 90 tablets (NDC 52427-435-90). ZESTORETIC 20-12.5 Tablets: White, round, biconvex, uncoated tablets identified with "142" debossed on one side and "ZESTORETIC" on the other side are supplied in bottles of 90 tablets (NDC 52427-436-90). ZESTORETIC 20-25 Tablets: Peach, round, biconvex, uncoated tablets identified with "145" debossed on one side and "ZESTORETIC" on the other side are supplied in bottles of 90 tablets (NDC 52427-437-90). ZESTORETIC is a trademark of Alvogen AZ IP Holdings LLC. Distributed by: Almatica Pharma, LLC., Morristown, NJ 07960 USA |
Drugs
| Drug | Countries | |
|---|---|---|
| ZESTORETIC | Canada, Cyprus, Spain, France, Ireland, Italy, Malta, Mexico, United Kingdom, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.