ZINGO Powder intradermal injection Ref.[49663] Active ingredients: Lidocaine
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
ZINGO (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate.
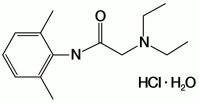
The chemical name is 2-diethylamino-2',6'-acetoxylidide, monohydrochloride, monohydrate. The molecular formula is C14H22N2O·HCl·H2O with a molecular weight of 288.8 Da. Lidocaine hydrochloride monohydrate, a local anesthetic of the amide class, has the following structural formula:
Lidocaine hydrochloride monohydrate is freely soluble in water, soluble in alcohol and chloroform, insoluble in ether, and melts at around 74-79°C.
ZINGO is a ready-to-use, sterile, single-use, disposable, needle-free delivery system. ZINGO consists of the following components: a drug reservoir cassette filled with 0.5 mg lidocaine hydrochloride monohydrate as a powder with a nominal particle size of 40 µm, a pressurized helium gas cylinder, and a safety interlock. The safety interlock prevents inadvertent actuation of the device. Once ZINGO is pressed against the skin, the interlock is released, allowing the button to be depressed to actuate the device. A sound similar to that of a popping balloon is emitted at the time ZINGO is actuated.
| Dosage Forms and Strengths |
|---|
|
ZINGO (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate. |
| How Supplied |
|---|
|
NDC 70645-123 ZINGO (lidocaine hydrochloride monohydrate) powder intradermal injection system contains 0.5 mg of sterile lidocaine hydrochloride monohydrate. ZINGO is a single-dose device packaged in an individual clear pouch. Twelve pouched devices are placed in labeled cartons. |
Drugs
| Drug | Countries | |
|---|---|---|
| ZINGO | Hong Kong, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.