ZINGO Powder intradermal injection Ref.[49663] Active ingredients: Lidocaine
Source: FDA, National Drug Code (US) Revision Year: 2018
1. Indications and Usage
ZINGO is indicated for use on intact skin to provide topical local analgesia prior to venipuncture or peripheral intravenous cannulation, in children 3-18 years of age.
ZINGO is indicated for use on intact skin to provide topical local analgesia prior to venipuncture in adults.
2. Dosage and Administration
Apply one ZINGO (0.5 mg lidocaine hydrochloride monohydrate) to the site planned for venipuncture or intravenous cannulation, one to three minutes prior to needle insertion.
Perform the procedure within 10 minutes after ZINGO administration.
Use ZINGO only on intact skin.
Application of one additional ZINGO at a new location is acceptable after a failed attempt at venous access. Multiple administrations of ZINGO at the same location are not recommended.
When ZINGO is used concomitantly with other products containing local anesthetic agents, the amount absorbed from all sources should be considered, as local anesthetics are thought to have at least additive toxicities.
2.1 Instructions for Use
Prepare the Treatment Site and Device
Examine the treatment site to ensure that the skin is intact. Clean the site, according to standard practice.
Visually inspect the pouch. Do not use if the pouch has been torn, or damaged or if the device has been dropped.
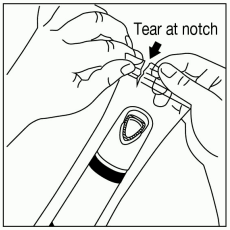
Tear open the pouch using the notch provided (Figure 1a). Remove ZINGO from the pouch, being careful not to touch the purple outlet (open end) to avoid contamination (Figure 1b).
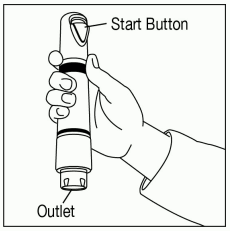
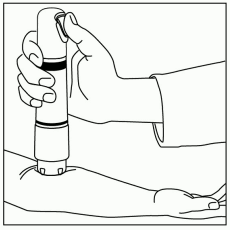
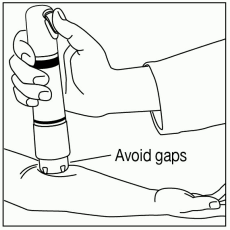
Position ZINGO: Grip ZINGO and place on the application site, with one hand, as illustrated in Figure 2, or with both hands, as shown in Figure 3.
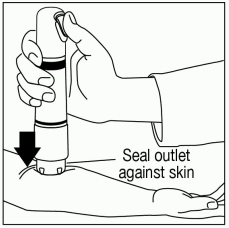
Ensure that the patient’s treatment site is supported to prevent movement. Seal the purple ZINGO outlet against the patient’s skin. Hold the device perpendicular to the skin, making sure that your thumb can reach the green start button.
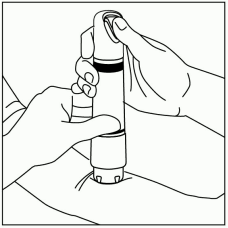
Avoid gaps between the skin and the ZINGO outlet, like the one illustrated in Figure 4, as gaps will impede drug delivery.
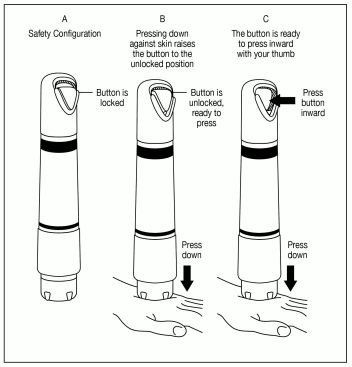
Release the Safety Interlock: Apply adequate downward pressure to release the safety interlock, while maintaining the seal between ZINGO and the skin.
ZINGO is ready for administration when the green start button has moved into the upward position, as illustrated in Figure 5a.
ZINGO cannot be actuated without releasing the internal safety interlock, as illustrated in Figure 5b.
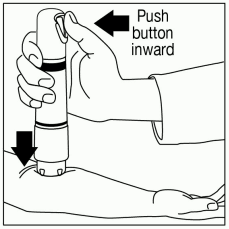
Administer ZINGO: While maintaining downward pressure, administer the dose by pressing the green start button, as illustrated in Figure 6. Do not move ZINGO during administration. Actuation is accompanied by a “popping” sound, indicating that the dose has been discharged.
Remove ZINGO: Remove ZINGO from the application site and dispose.
Begin Procedure: Start the venipuncture or intravenous cannulation procedure 1-3 minutes after ZINGO administration.
10. Overdosage
In adults following a single administration of ZINGO the plasma levels of lidocaine were below the limit of detection (5 ng/mL). Signs of central nervous system (CNS) toxicity may start at plasma concentrations of lidocaine as low as 1000 ng/mL, and the risk of seizures generally increases with increasing plasma levels. Very high levels of lidocaine can cause respiratory arrest, coma, decreases in cardiac output, total peripheral resistance, and mean arterial pressure, ventricular arrhythmias, and cardiac arrest. The toxicity of coadministered local anesthetics is thought to be at least additive. In the absence of massive topical overdose or oral ingestion, other etiologies for the clinical effects or overdosage from other sources of lidocaine or other local anesthetics should be considered. The management of overdosage includes close monitoring, supportive care, and symptomatic treatment. Dialysis is of negligible value in the treatment of acute overdosage of lidocaine.
16.2. Storage and Handling
Cartons are stored at 20° to 25°C (68° to 77°F); excursions permitted between 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.