CONTRAVE Extended-release tablet Ref.[50756] Active ingredients: Bupropion Naltrexone
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
CONTRAVE extended-release tablets contain naltrexone hydrochloride (HCl) and bupropion HCl.
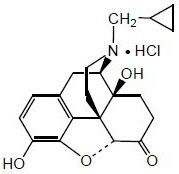
Naltrexone HCl, USP, an opioid antagonist, is a synthetic congener of oxymorphone with no opioid agonist properties. Naltrexone differs in structure from oxymorphone in that the methyl group on the nitrogen atom is replaced by a cyclopropylmethyl group. Naltrexone HCl is also related to the potent opioid antagonist, naloxone, or n-allylnoroxymorphone.
Naltrexone HCl has the chemical name of morphinan-6-one, 17-(cyclopropylmethyl)-4,5-epoxy-3,14-dihydroxy-, hydrochloride, (5α)-. The empirical formula is C20H23NO4•HCl and the molecular weight is 377.86.
The structural formula is:
Naltrexone HCl is a white to yellowish, crystalline compound. It is soluble in water to the extent of about 100 mg/mL.
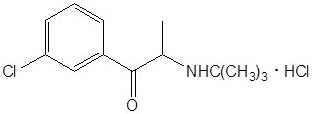
Bupropion HCl is an antidepressant of the aminoketone class. Bupropion HCl closely resembles the structure of diethylpropion. It is designated as (±)-1-(3 chlorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propranone hydrochloride. It is related to phenylethylamines. The empirical formula is C13H18ClNO•HCl and the molecular weight is 276.2.
The structural formula is:
Bupropion HCl powder is white, crystalline, and highly soluble in water.
CONTRAVE is available for oral administration as a round, bi-convex, film-coated, extended-release tablet. Each tablet has a trilayer core composed of two drug layers, containing the drug and excipients, separated by a more rapidly dissolving inert layer. Each tablet contains 8 mg of naltrexone HCl and 90 mg of bupropion HCl. Tablets are blue and are debossed with NB-890 on one side. Each tablet contains the following inactive ingredients: microcrystalline cellulose, hydroxypropyl cellulose, lactose anhydrous, L-cysteine hydrochloride, crospovidone, magnesium stearate, hypromellose, edetate disodium, lactose monohydrate, colloidal silicon dioxide, Opadry II Blue and FD&C Blue #2 aluminum lake.
| Dosage Forms and Strengths |
|---|
|
CONTRAVE extended-release tablets: 8 mg/90 mg are blue, round, bi-convex, film-coated, and debossed with "NB-890" on one side. |
| How Supplied | ||
|---|---|---|
|
CONTRAVE 8 mg/90 mg (naltrexone HCl 8 mg and bupropion HCl 90 mg) extended-release tablets are blue, round, bi-convex, film-coated tablets debossed with "NB-890" on one side. CONTRAVE tablets are available as follows:
Distributed by: Currax Pharmaceuticals LLC, Brentwood, TN 37027 |
Drugs
| Drug | Countries | |
|---|---|---|
| CONTRAVE | Canada, Ecuador, Hong Kong, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.