CONTRAVE Extended-release tablet Ref.[50756] Active ingredients: Bupropion Naltrexone
Source: FDA, National Drug Code (US) Revision Year: 2022
12.1. Mechanism of Action
CONTRAVE has two components: naltrexone, an opioid antagonist, and bupropion, a relatively weak inhibitor of the neuronal reuptake of dopamine and norepinephrine. Nonclinical studies suggest that naltrexone and bupropion have effects on two separate areas of the brain involved in the regulation of food intake: the hypothalamus (appetite regulatory center) and the mesolimbic dopamine circuit (reward system). The exact neurochemical effects of CONTRAVE leading to weight loss are not fully understood.
12.2. Pharmacodynamics
Combined, bupropion and naltrexone increased the firing rate of hypothalamic pro-opiomelanocortin (POMC) neurons in vitro, which are associated with regulation of appetite. The combination of bupropion and naltrexone also reduced food intake when injected directly into the ventral tegmental area of the mesolimbic circuit in mice, an area associated with regulation of reward pathways.
Cardiac Electrophysiology
At the recommended dose, CONTRAVE does not prolong the QTc interval to any clinically relevant extent.
12.3. Pharmacokinetics
Absorption
Naltrexone
Following single oral administration of CONTRAVE (two 8 mg naltrexone/90 mg bupropion tablets) to healthy subjects, mean peak naltrexone concentration (Cmax) was 1.4 ng/mL, time to peak concentration (Tmax) was 2 hours, and extent of exposure (AUC0-inf) was 8.4 ng∙hr/mL.
Bupropion
Following single oral administration of CONTRAVE (two 8 mg naltrexone/90 mg bupropion tablets) to healthy subjects, mean peak bupropion concentration (Cmax) was 168 ng/mL, time to peak concentration (Tmax) was three hours, and extent of exposure (AUC0-inf) was 1,607 ng∙hr/mL.
Food Effect on Absorption
When CONTRAVE was administered with a high-fat meal, the AUC and Cmax for naltrexone increased 2.1-fold and 3.7-fold, respectively, and the AUC and Cmax for bupropion increased 1.4-fold and 1.8-fold, respectively. At steady state, the food effect increased AUC and Cmax for naltrexone by 1.7-fold and 1.9-fold, respectively, and increased AUC and Cmax for bupropion by 1.1-fold and 1.3-fold, respectively. Thus, CONTRAVE should not be taken with high-fat meals because of the resulting significant increases in bupropion and naltrexone systemic exposure.
Distribution
Naltrexone
Naltrexone is 21% plasma protein bound. The mean apparent volume of distribution at steady state for naltrexone (Vss/F) is 5,697 liters.
Bupropion
Bupropion is 84% plasma protein bound. The mean apparent volume of distribution at steady state for bupropion (Vss/F) is 880 liters.
Metabolism and Excretion
Naltrexone
The major metabolite of naltrexone is 6-beta-naltrexol. The activity of naltrexone is believed to be the result of both the parent and the 6-beta-naltrexol metabolite. Though less potent, 6-beta-naltrexol is eliminated more slowly and thus circulates at much higher concentrations than naltrexone. Naltrexone and 6-beta-naltrexol are not metabolized by cytochrome P450 enzymes and in vitro studies indicate that there is no potential for inhibition or induction of important isozymes.
Naltrexone and its metabolites are excreted primarily by the kidney (53% to 79% of the dose). Urinary excretion of unchanged naltrexone accounts for less than 2% of an oral dose. Urinary excretion of unchanged and conjugated 6-beta-naltrexol accounts for 43% of an oral dose. The renal clearance for naltrexone ranges from 30 to 127 mL/min, suggesting that renal elimination is primarily by glomerular filtration. The renal clearance for 6-beta-naltrexol ranges from 230 to 369 mL/min suggesting an additional renal tubular secretory mechanism. Fecal excretion is a minor elimination pathway.
Following single oral administration of CONTRAVE tablets to healthy subjects, mean elimination half-life (T1/2) was approximately 5 hours for naltrexone. Following twice daily administration of CONTRAVE, naltrexone did not accumulate and its kinetics appeared linear. However, in comparison to naltrexone, 6-beta-naltrexol accumulates to a larger extent (accumulation ratio ~3).
Bupropion
Bupropion is extensively metabolized with three active metabolites: hydroxybupropion, threohydrobupropion and erythrohydrobupropion. The metabolites have longer elimination half-lives than bupropion and accumulate to a greater extent. Following bupropion administration, more than 90% of the exposure is a result of metabolites. In vitro findings suggest that CYP2B6 is the principal isozyme involved in the formation of hydroxybupropion whereas cytochrome P450 isozymes are not involved in the formation of the other active metabolites. Bupropion and its metabolites inhibit CYP2D6. Plasma protein binding of hydroxybupropion is similar to that of bupropion (84%) whereas the other two metabolites have approximately half the binding.
Following oral administration of 200 mg of 14C-bupropion in humans, 87% and 10% of the radioactive dose were recovered in the urine and feces, respectively. The fraction of the oral dose of bupropion excreted unchanged was 0.5%, a finding consistent with the extensive metabolism of bupropion.
Following single oral administration of CONTRAVE tablets to healthy subjects, mean elimination half-life (T½) was approximately 21 hours for bupropion. Following twice daily administration of CONTRAVE, metabolites of bupropion, and to a lesser extent unchanged bupropion, accumulate and reach steady-state concentrations in approximately one week.
Specific Populations
Gender
Pooled analysis of CONTRAVE data suggested no clinically meaningful differences in the pharmacokinetic parameters of bupropion or naltrexone based on gender.
Race
Pooled analysis of CONTRAVE data suggested no clinically meaningful differences in the pharmacokinetic parameters of bupropion or naltrexone based on race.
Elderly
The pharmacokinetics of CONTRAVE have not been evaluated in the geriatric population. The effects of age on the pharmacokinetics of naltrexone or bupropion and their metabolites have not been fully characterized. An exploration of steady-state bupropion concentrations from several depression efficacy studies involving patients dosed in a range of 300 to 750 mg/day, on a three times daily schedule, revealed no relationship between age (18 to 83 years) and plasma concentration of bupropion. A single-dose pharmacokinetic study demonstrated that the disposition of bupropion and its metabolites in elderly subjects was similar to that of younger subjects. These data suggest there is no prominent effect of age on bupropion concentration; however, another pharmacokinetic study, single and multiple dose, has suggested that the elderly are at increased risk for accumulation of bupropion and its metabolites [see Use in Specific Populations (8.5)].
Smokers
Pooled analysis of CONTRAVE data revealed no meaningful differences in the plasma concentrations of bupropion or naltrexone in smokers compared with nonsmokers. The effects of cigarette smoking on the pharmacokinetics of bupropion were studied in 34 healthy male and female volunteers; 17 were chronic cigarette smokers and 17 were nonsmokers. Following oral administration of a single 150 mg dose of bupropion, there was no statistically significant difference in Cmax, half-life, Tmax, AUC, or clearance of bupropion or its active metabolites between smokers and nonsmokers.
Hepatic Impairment
A single dose pharmacokinetic study conducted for CONTRAVE, comparing patients with mild (n=8), moderate (n=8) and severe (n=7) hepatic impairment based on CHILD-PUGH classification system to subjects with normal hepatic function (n=13), showed that hepatic impairment had a significant effect on the PK parameters of the parent drugs naltrexone and bupropion. Systemic exposure to some metabolites was also increased in patients with impaired hepatic function [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)].
Following a single dose of naltrexone/bupropion, AUCinf of naltrexone was approximately 2.8-, 6.1-, and 34-fold higher in patients with mild, moderate, and severe hepatic impairment, respectively. In patients with moderate and severe hepatic impairment, AUCinf of bupropion was approximately 2.0- and 3.6-fold higher, respectively, compared to subjects with normal hepatic function. There was no effect of mild hepatic impairment on bupropion exposure. Exposure to bupropion metabolite, threohydrobupropion, was increased by 1.9-, 3.4-, and 2.5-fold in patients with mild, moderate, and severe hepatic impairment, respectively [see Dosage and Administration (2.3) and Use in Specific Populations (8.7)].
Renal Impairment
A single-dose pharmacokinetic study conducted for CONTRAVE, comparing patients with mild (n=8), moderate (n=8) and severe (n=7) renal impairment to subjects with normal renal function (n=13), showed that renal impairment had no significant effect on the PK parameters of the parent drugs naltrexone and bupropion. However, systemic exposure (AUCinf) of some metabolites was increased in patients with impairment of renal function [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
Following a single-dose of 16 mg naltrexone /180 mg bupropion, AUCinf of 6-beta-naltrexol was approximately1.5-, 1.7-, and 2.2-fold higher in patients with mild, moderate, and severe renal impairment, respectively. In patients with mild, moderate, and severe renal impairment, AUC of bupropion metabolites threohydrobupropion and erythrohydrobupropion increased approximately 1.3-, 1.9-, and 1.7-fold and 1.2-, 1.8-, and 1.5-fold, respectively. No studies have been conducted for CONTRAVE in patients with end stage renal disease.
The following information is available for individual components.
In a study of seven patients with end-stage renal disease requiring dialysis, peak plasma concentrations of naltrexone were elevated at least 6-fold compared to healthy subjects. An inter-trial comparison between normal subjects and patients with end-stage renal failure demonstrated that the bupropion Cmax and AUC values were comparable in the two groups, whereas the hydroxybupropion and threohydrobupropion metabolites had a 2.3- and 2.8-fold increase, respectively, in AUC for patients with end-stage renal failure.
Drug Interactions
In vitro Assessment of Drug Interactions
At therapeutically relevant concentrations, naltrexone and 6-beta-naltrexol are not major inhibitors of CYP isoforms CYP1A2, CYP2B6, CYP2C8, CYP2E1, CYP2C9, CYP2C19, CYP2D6 or CYP3A4. Both naltrexone and 6-beta-naltrexol are not major inducers of CYP isoforms CYP1A2, CYP2B6, or CYP3A4.
Bupropion and its metabolites (hydroxybupropion, erythrohydrobupropion, threohydrobupropion) are inhibitors of CYP2D6.
In vitro studies suggest that paroxetine, sertraline, norfluoxetine, fluvoxamine, and nelfinavir inhibit the hydroxylation of bupropion.
Bupropion (IC50 9.3 mcM) and its metabolites, hydroxybupropion (IC50 82 mcM) and threohydrobupropion and erythrohydrobupropion (1:1 mixture; IC50 7.8 mcM), inhibited the renal organic transporter OCT2 to a clinically relevant level.
Effects of Naltrexone/Bupropion on the Pharmacokinetics of Other Drugs
Drug interaction between CONTRAVE and CYP2D6 substrates (metoprolol) or other drugs (atorvastatin, glyburide, lisinopril, nifedipine, valsartan) has been evaluated. In addition, drug interaction between bupropion, a component of CONTRAVE, and CYP2D6 substrates (desipramine) or other drugs (citalopram, lamotrigine) has also been evaluated.
Table 4. Effect of Naltrexone/Bupropion Coadministration on Systemic Exposure of Other Drugs:
| Naltrexone/Bupropion Dosage | Coadministered Drug | |
|---|---|---|
| Name and Dose Regimens | Change in Systemic Exposure | |
| Initiate the following drugs at the lower end of the dose range during concomitant use with CONTRAVE [see Drug Interactions 7]: | ||
| Bupropion 150 mg twice daily for 10 days | Desipramine 50 mg single dose | ↑5-fold AUC, ↑2-fold Cmax |
| Bupropion 300 mg (as XL) once daily for 14 days | Citalopram 40 mg once daily for 14 days | ↑40% AUC, ↑30% Cmax |
| Naltrexone/Bupropion 16 mg/180 mg twice daily for 7 days | Metoprolol 50 mg single dose | ↑4-fold AUC, ↑2-fold Cmax |
| No dose adjustment needed for the following drugs during concomitant use with CONTRAVE: | ||
| Naltrexone/Bupropion 16 mg/180 mg single dose | Atorvastatin 80 mg single dose | No Effect |
| Naltrexone/Bupropion 16 mg/180 mg single dose | Glyburide 6 mg single dose | No Effect |
| Naltrexone/Bupropion 16 mg/180 mg single dose | Lisinopril 40 mg single dose | No Effect |
| Naltrexone/Bupropion 16 mg/180 mg twice daily | Metformin 850 mg single dose | ↑23% AUC; No effect on Cmax |
| Naltrexone/Bupropion 16 mg/180 mg single dose | Nifedipine 90 mg single dose | No Effect |
| Naltrexone/Bupropion 16 mg/180 mg single dose | Valsartan 320 mg single dose | No Effect |
| Bupropion 150 mg twice daily for 12 days | Lamotrigine 100 mg single dose | No Effect |
Digoxin: Literature data showed that digoxin exposure was decreased when a single oral dose of 0.5 mg digoxin was administered 24 hours after a single oral dose of extended-release 150 mg bupropion in healthy volunteers.
Effects of Other Drugs on the Pharmacokinetics of Naltrexone/Bupropion
Drug interactions between CYP2B6 inhibitors (ticlopidine, clopidogrel, prasugrel), CYP2B6 inducers (ritonavir, lopinavir) and bupropion (one of the CONTRAVE components), or between other drugs (atorvastatin, glyburide, metoprolol, lisinopril, nifedipine, valsartan) and CONTRAVE have been evaluated. While not systematically studied, carbamazepine, phenobarbital, or phenytoin may induce the metabolism of bupropion.
Table 5. Effect of Coadministered Drugs on Systemic Exposure of Naltrexone/Bupropion:
| Name and Dose Regimens | Coadministered Drug | |
|---|---|---|
| CONTRAVE Components | Change in Systemic Exposure | |
| Do not exceed one tablet twice daily dose of CONTRAVE with the following drugs: | ||
| Ticlopidine 250 mg twice daily for 4 days | Bupropion Hydroxybupropion | ↑85% AUC, ↑38% Cmax ↓84% AUC, ↓78% Cmax |
| Clopidogrel 75 mg once daily for 4 days | Bupropion Hydroxybupropion | ↑60% AUC, ↑40% Cmax ↓52% AUC, ↓50% Cmax |
| No dose adjustment needed for CONTRAVE with the following drugs: | ||
| Atorvastatin 80 mg single dose | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | No Effect No Effect No Effect No Effect No Effect No Effect |
| Lisinopril 40 mg single dose | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | No Effect No Effect No Effect No Effect No Effect No Effect |
| Valsartan 320 mg single dose | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | No Effect No Effect No Effect ↓14% AUC, No Effect on Cmax No Effect No Effect |
| Cimetidine 800 mg single dose | Bupropion Hydroxybupropion Threo/Erythrohydrobupropion | No Effect No Effect ↑16% AUC, ↑32% Cmax |
| Citalopram 40 mg once daily for 14 days | Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | No Effect No Effect No Effect No Effect |
| Metoprolol 50 mg single dose | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | ↓25% AUC, ↓29% Cmax No Effect No Effect No Effect No Effect No Effect |
| Metformin 850 mg single dose | Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion Naltrexone 6-beta naltrexol | No Effect No Effect No Effect No Effect No Effect No Effect |
| Nifedipine 90 mg single dose | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | ↑24% AUC, ↑58% Cmax No Effect No Effect on AUC, ↑22% Cmax No Effect No Effect No Effect |
| Prasugrel 10 mg once daily for 6 days | Bupropion Hydroxybupropion | ↑18% AUC, ↑14% Cmax ↓24%AUC, ↓32% Cmax |
| Use CONTRAVE with caution with the following drugs: | ||
| Glyburide 6 mg single dose* | Naltrexone 6-beta naltrexol Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | ↑2-fold AUC, ↑2-fold Cmax No Effect ↑36% AUC, ↑18% Cmax ↑22% AUC, ↑21% Cmax No Effect on AUC, ↑15% Cmax No Effect |
| Avoid concomitant use of CONTRAVE with following drugs: | ||
| Ritonavir 100 mg twice daily for 17 days | Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | ↓22% AUC, ↓21 % Cmax ↓23% AUC, No Effect on Cmax ↓38% AUC, ↓39 % Cmax ↓48% AUC, ↓28 % Cmax |
| 600 mg twice daily for 8 days | Bupropion Hydroxybupropion Threohydrobupropion Erythrohydrobupropion | ↓66% AUC, ↓62% Cmax ↓78% AUC, ↓42 % Cmax ↓50% AUC, ↓58% Cmax ↓68% AUC, ↓48 % Cmax |
| Lopinavir/Ritonavir 400 mg/100 mg twice daily for 14 days | Bupropion Hydroxybupropion | ↓57% AUC, ↓57% Cmax ↓50% AUC, ↓31% Cmax |
| Efavirenz 600 mg once daily for 2 weeks | Bupropion Hydroxybupropion | ↓55% AUC, ↓34% Cmax No Effect on AUC, ↑50% Cmax |
* Results were confounded by the food-effect due to oral glucose coadministered with the treatment.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies to evaluate carcinogenesis, mutagenesis, or impairment of fertility with the combined products in CONTRAVE have not been conducted. The following findings are from studies performed individually with naltrexone and bupropion. The potential carcinogenic, mutagenic and fertility effects of the metabolite 6-beta-naltrexol are unknown. Safety margins were estimated using body surface area exposure (mg/m²) based on a body weight of 100 kg.
In a two-year carcinogenicity study in rats with naltrexone, there were small increases in the numbers of testicular mesotheliomas in males and tumors of vascular origin in males and females. The incidence of mesothelioma in males given naltrexone at a dietary dose of 100 mg/kg/day (approximately 50 times the recommended therapeutic dose on a mg/m² basis for the naltrexone maintenance dose for CONTRAVE) was 6%, compared with a maximum historical incidence of 4%. The incidence of vascular tumors in males and females given dietary doses of 100 mg/kg/day was 4%, but only the incidence in females was increased compared with a maximum historical control incidence of 2%. There was no evidence of carcinogenicity in a two-year dietary study with naltrexone in male and female mice.
Lifetime carcinogenicity studies of bupropion were performed in rats and mice at doses up to 300 and 150 mg/kg/day, respectively. These doses are approximately 14 and 3 times the maximum recommended human dose (MRHD) of the bupropion component in CONTRAVE, respectively, on a mg/m² basis. In the rat study there was an increase in nodular proliferative lesions of the liver at doses of 100 to 300 mg/kg/day (approximately 5 to 14 times the MRHD of the bupropion component in CONTRAVE on a mg/m² basis); lower doses were not tested. The question of whether or not such lesions may be precursors of neoplasms of the liver is currently unresolved. Similar liver lesions were not seen in the mouse study, and no increase in malignant tumors of the liver and other organs was seen in either study.
There was limited evidence of a weak genotoxic effect of naltrexone in one gene mutation assay in a mammalian cell line, in the Drosophila recessive lethal assay, and in non-specific DNA repair tests with E. coli. However, no evidence of genotoxic potential was observed in a range of other in vitro tests, including assays for gene mutation in bacteria, yeast, or in a second mammalian cell line, a chromosomal aberration assay, and an assay for DNA damage in human cells. Naltrexone did not exhibit clastogenicity in an in vivo mouse micronucleus assay.
Bupropion produced a positive response (two to three times control mutation rate) in two of five strains in the Ames bacterial mutagenicity test and an increase in chromosomal aberrations in one of three in vivo rat bone marrow cytogenetic studies.
Naltrexone administered orally to rats caused a significant increase in pseudopregnancy and a decrease in pregnancy rates in rats at 100 mg/kg/day (approximately 50 times the MRHD of the naltrexone component in CONTRAVE on a mg/m² basis). There was no effect on male fertility at this dose level. The relevance of these observations to human fertility is not known.
A fertility study of bupropion in rats at doses up to 300 mg/kg/day (approximately 14 times the MRHD of the bupropion component in CONTRAVE on a mg/m² basis) revealed no evidence of impaired fertility.
14. Clinical Studies
The effects of CONTRAVE on weight loss in conjunction with reduced caloric intake and increased physical activity was studied in double-blind, placebo-controlled trials (BMI range 27 to 45 kg/m²) with study durations of 16 to 56 weeks randomized to naltrexone and/or bupropion or placebo.
Effect on Weight Loss and Weight Maintenance
Four 56-week multicenter, double-blind, placebo-controlled obesity trials (CONTRAVE Obesity Research, or COR-I, COR-II, COR-BMOD, and COR-Diabetes) were conducted to evaluate the effect of CONTRAVE in conjunction with lifestyle modification in 4,536 patients randomized to CONTRAVE or placebo. The COR-I, COR-II, and COR-BMOD trials enrolled patients with obesity (BMI 30 kg/m² or greater) or overweight (BMI 27 kg/m² or greater) and at least one comorbidity (hypertension or dyslipidemia). The COR-Diabetes trial enrolled patients with BMI greater than 27 kg/m² with type 2 diabetes with or without hypertension and/or dyslipidemia.
Treatment was initiated with a three-week dose-escalation period followed by approximately 1 year of continued therapy. Patients were instructed to take CONTRAVE with food. COR-I and COR-II included a program consisting of a reduced-calorie diet resulting in an approximate 500 kcal/day decrease in caloric intake, behavioral counseling, and increased physical activity. COR-BMOD included an intensive behavioral modification program consisting of 28 group counseling sessions over 56 weeks as well as a prescribed diet and exercise regimen. COR-Diabetes evaluated patients with type 2 diabetes not achieving glycemic goal of a HbA1c less than 7% either with oral antidiabetic agents or with diet and exercise alone. Of the overall population from these four trials, 24% had hypertension, 54% had dyslipidemia at study entry, and 10% had type 2 diabetes.
Apart from COR-Diabetes, which only enrolled patients with type 2 diabetes, the demographic characteristics of patients were similar across all four trials. For the four trial populations combined, the mean age was 46 years, 83% were female, 77% were Caucasian, 18% were black, and 5% were other races. At baseline, mean BMI was 36 kg/m² and mean waist circumference was 110 cm.
A substantial percentage of randomized patients withdrew from the trials prior to Week 56: 45% for the placebo group and 46% for the CONTRAVE group. The majority of these patients discontinued within the first 12 weeks of treatment. Approximately 24% of patients treated with CONTRAVE and 12% of patients treated with placebo discontinued treatment because of an adverse reaction [see Adverse Reactions (6.1)].
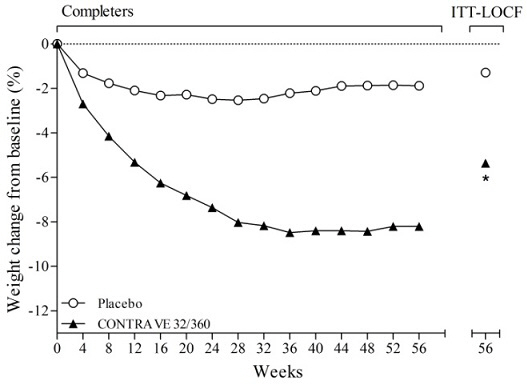
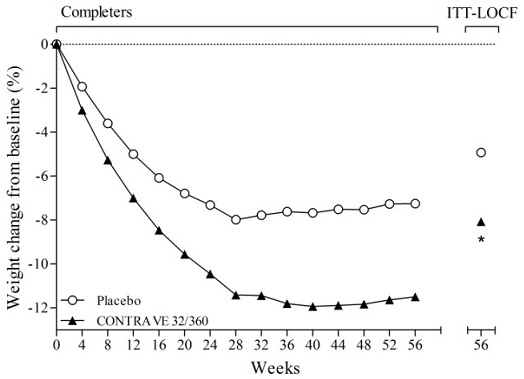
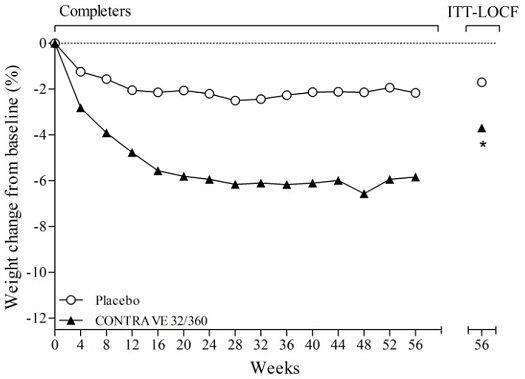
The co-primary endpoints were percent change from baseline body weight and the proportion of patients achieving at least a 5% reduction in body weight. In the 56-week COR-I trial, the mean change in body weight was -5.4% among patients assigned to CONTRAVE 32 mg/360 mg compared with -1.3% among patients assigned to placebo (Intent-To-Treat [ITT] population), as shown in Table 6 and Figure 1. In this trial, the achievement of at least a 5% reduction in body weight from baseline occurred more frequently for patients treated with CONTRAVE 32 mg/360 mg compared with placebo (42% vs 17%; Table 6). Results from COR-BMOD and COR-Diabetes are shown in Table 6 and Figures 2 and 3.
Table 6. Changes in Weight in 56-Week Trials with CONTRAVE (ITT/LOCF*):
| COR-I | COR-BMOD | COR-Diabetes | ||||
|---|---|---|---|---|---|---|
| CONTRAVE 32 mg/ 360 mg | Placebo | CONTRAVE 32 mg/ 360 mg | Placebo | CONTRAVE 32 mg/ 360 mg | Placebo | |
| N | 538 | 536 | 565 | 196 | 321 | 166 |
| Weight (kg) | ||||||
| Baseline mean (SD) | 99.8 (16.1) | 99.5 (14.4) | 100.3 (15.5) | 101.8 (15.0) | 104.2 (19.1) | 105.3 (16.9) |
| LS Mean % Change From Baseline (SE) | -5.4 (0.3) | -1.3 (0.3) | -8.1 (0.4) | -4.9 (0.6) | -3.7 (0.3) | -1.7 (0.4) |
| Difference from placebo (95% CI) | -4.1† (-4.9, -3.3) | -3.2† (-4.5, -1.8) | -2.0† (-3.0, -1.0) | |||
| Percentage of patients losing greater than or equal to 5% body weight | 42 | 17 | 57 | 43 | 36 | 18 |
| Risk difference vs placebo (95% CI) | 25† (19, 30) | 14† (6, 22) | 18† (9, 25) | |||
| Percentage of patients losing greater than or equal to 10% body weight | 21 | 7 | 35 | 21 | 15 | 5 |
| Risk difference vs placebo (95% CI) | 14† (10, 18) | 14† (7, 21) | 10‡ (4, 15) | |||
Type 1 error was controlled across all 3 endpoints
* Based on last observation carried forward (LOCF) in all randomized subjects who had a baseline body weight measurement and at least one post baseline body weight measurement during the defined treatment phase. All available body weight data during the double-blind treatment phase are included in the analysis, including data collected from subjects who discontinued study drug.
† Difference from placebo, p<0.001
‡ Difference from placebo, p<0.01
The percentages of patients who achieved at least 5% or at least 10% body weight loss from baseline were greater among those assigned to CONTRAVE, compared with placebo, in all four obesity trials (Table 6).
Figure 1. Weight Loss Over Time in Completer
Population: COR-I Trial:
* p<0.001 vs placebo
COR-I trial: 50.1% in the placebo group and 49.2% in the CONTRAVE group discontinued study drug.
Figure 2. Weight Loss Over Time in Completer
Population: COR-BMOD Trial:
* p<0.001 vs placebo
COR-BMOD trial: 41.6% in the placebo group and 42.1% in the CONTRAVE group discontinued study drug.
Figure 3. Weight Loss Over Time in Completer
Population: COR-Diabetes Trial:
* p<0.001 vs placebo
COR-Diabetes trial: 41.2% in the placebo group and 47.8% in the CONTRAVE group discontinued study drug.
Effect on Cardiovascular and Metabolic Parameters
Changes in cardiovascular and metabolic parameters associated with obesity are presented for COR-I and COR-BMOD (Table 7). Changes in mean blood pressure and heart rate are further described elsewhere [see Warnings and Precautions (5.5)].
Table 7. Change in Markers of Cardiovascular and Metabolic Parameters from Baseline in 56 Week Trials with CONTRAVE 32 mg/360 mg (COR-I and COR-BMOD)*:
| Parameter | COR-I | COR-BMOD | ||||
|---|---|---|---|---|---|---|
| CONTRAVE 32 mg/360 mg N=471 | Placebo N=511 | CONTRAVE minus Placebo (LS Mean) | CONTRAVE 32 mg/360 mg N=482 | Placebo N=193 | CONTRAVE minus Placebo (LS Mean) | |
| Triglycerides, mg/dL | ||||||
| Baseline median (Q1, Q3) | 113 (86, 158) | 112 (78, 157) | -10.7† | 110 (78, 162) | 103 (76, 144) | -9.9† |
| Median % change | -11.6 | 1.7 | -17.8 | -7.4 | ||
| HDL-C, mg/dL | ||||||
| Baseline mean (SD) | 51.9 (13.6) | 52.0 (13.6) | 7.2 | 53.6 (13.5) | 55.3 (12.9) | 6.6 |
| LS Mean % change (SE) | 8.0 (0.9) | 0.8 (0.9) | 9.4 (1.0) | 2.8 (1.6) | ||
| LDL-C, mg/dL | ||||||
| Baseline mean (SD) | 118.8 (32.6) | 119.7 (34.8) | -1.5 | 109.5 (27.5) | 109.2 (27.3) | -2.9 |
| LS Mean % change (SE) | -2.0 (1.0) | -0.5 (1.1) | 7.1 (1.4) | 10.0 (2.2) | ||
| Waist circumference, cm | ||||||
| Baseline mean (SD) | 108.8 (11.3) | 110.0 (12.2) | -3.8‡ | 109.3 (11.4) | 109.0 (11.8) | -3.2‡ |
| LS Mean change (SE) | -6.2 (0.4) | -2.5 (0.4) | -10.0 (0.5) | -6.8 (0.8) | ||
| Heart rate, bpm | ||||||
| Baseline mean (SD) | 72.1 (8.7) | 71.8 (8.0) | 1.2 | 70.7 (8.3) | 70.4 (9.0) | 0.9 |
| LS Mean change (SE) | 1.0 (0.3) | -0.2 (0.3) | 1.1 (0.4) | 0.2 (0.5) | ||
| Systolic blood pressure, mmHg | ||||||
| Baseline mean (SD) | 118.9 (9.8) | 119.0 (9.8) | 1.8 | 116.9 (9.9) | 116.7 (10.9) | 2.6 |
| LS Mean change (SE) | -0.1 (0.4) | -1.9 (0.4) | -1.3 (0.5) | -3.9 (0.7) | ||
| Diastolic blood pressure, mmHg | ||||||
| Baseline mean (SD) | 77.1 (7.2) | 77.3 (6.6) | 0.9 | 78.2 (7.2) | 77.2 (7.4) | 1.4 |
| LS Mean change (SE) | 0.0 (0.3) | -0.9 (0.3) | -1.4 (0.3) | -2.8 (0.5) | ||
Q1: first quartile; Q3: third quartile
*Based on last observation carried forward (LOCF) while on study drug
† Hodges-Lehmann estimate of treatment difference
‡ Statistically significant vs placebo (p<0.001) based on the pre-specified closed testing procedure method for controlling Type I error
Effect of CONTRAVE on Cardiometabolic Parameters and Anthropometry in Patients with Type 2 Diabetes Mellitus
Changes in glycemic control observed from baseline to Week 56 among patients with type 2 diabetes and obesity assigned to either CONTRAVE 32 mg/360 mg or placebo are shown in Table 8.
Table 8. Changes in Cardiometabolic Parameters and Waist Circumference in Patients with Type 2 Diabetes Mellitus in a 56 Week Trial with CONTRAVE 32 mg/360 mg (COR-Diabetes):
| CONTRAVE 32 mg/360 mg N=265 | Placebo N=159 | CONTRAVE minus Placebo (LS Mean) | |||
| Baseline | Change from Baseline (LS Mean) | Baseline | Change from Baseline (LS Mean) | ||
| HbA1c (%) | 8.0 | -0.6 | 8.0 | -0.1 | -0.5* |
| Fasting Glucose (mg/dL) | 160.0 | -11.9 | 163.9 | -4.0 | -7.9 |
| Waist Circumference (cm) | 115.6 | -5.0 | 114.3 | -2.9 | -2.1 |
| Systolic blood pressure (mmHg) | 125.0 | 0.0 | 124.5 | -1.1 | 1.2 |
| Diastolic blood pressure (mmHg) | 77.5 | -1.1 | 77.4 | -1.5 | 0.4 |
| Heart rate (bpm) | 72.9 | 0.7 | 73.1 | -0.2 | 0.9 |
| Baseline | % Change from Baseline (LS Mean) | Baseline | % Change from Baseline (LS Mean) | CONTRAVE minus Placebo (LS Mean) | |
| Triglycerides (mg/dL)† | 147 (98, 200) | -7.7 | 168 (114, 236) | -8.6 | -3.3 |
| HDL Cholesterol (mg/dL) | 46.2 | 7.4 | 46.1 | -0.2 | 7.6 |
| LDL Cholesterol (mg/dL) | 100.2 | 2.4 | 101.0 | 4.2 | -1.9 |
Based on last observation carried forward (LOCF) while on study drug
* Statistically significant vs placebo (p<0.001) based on the pre-specified closed testing procedure method for controlling Type I error
† Values are baseline median (first and third quartiles), median % change, and the Hodges-Lehmann estimate of the median treatment difference
Effect on Body Composition
In a subset of 124 patients (79 CONTRAVE, 45 placebo), body composition was measured using dual energy X-ray absorptiometry (DEXA). The DEXA assessment showed that mean total body fat mass decreased by 4.7 kg (11.7%) in the CONTRAVE group vs 1.4 kg (4.3%) in the placebo group at Week 52/LOCF (treatment difference, -3.3 kg [-7.4%], p<0.01).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.