NORGESIC FORTE Tablet Ref.[50735] Active ingredients: Acetylsalicylic acid Caffeine Orphenadrine
Source: FDA, National Drug Code (US) Revision Year: 2022
Product description
Each Norgesic Forte tablet, for oral administration, contains Orphenadrine Citrate 50 mg, Aspirin 770 mg, Caffeine 60 mg.
In addition, each tablet contains the following inactive ingredients, anhydrous lactous, colloidal silicon dioxide, D&C yellow #10, FD&C blue #1, zinc stearate, providone, pregetanized starch, and stearic acid.
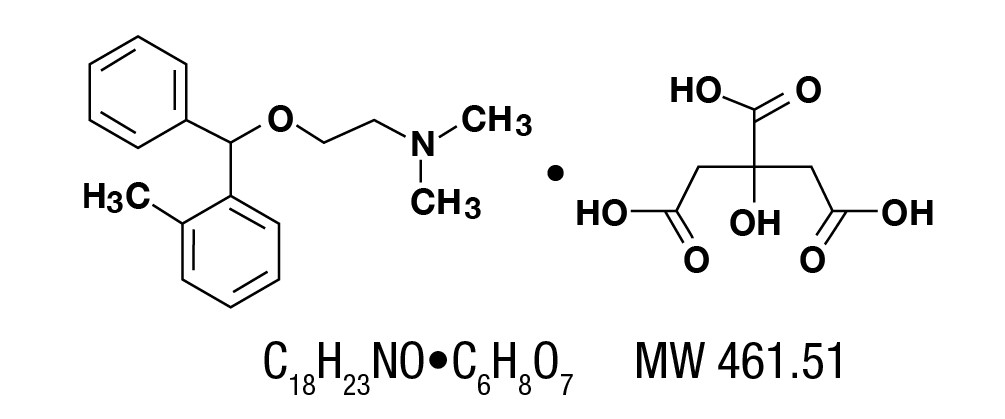
Orphenadrine citrate, (2-dimethylaminoethyl 2-methybenzhydryl ether citrate). It is as a white, practically odorless, crystalline powder, having a bitter taste. It is sparingly soluble in water, slightly soluble in alcohol.
It has the following structural formula:
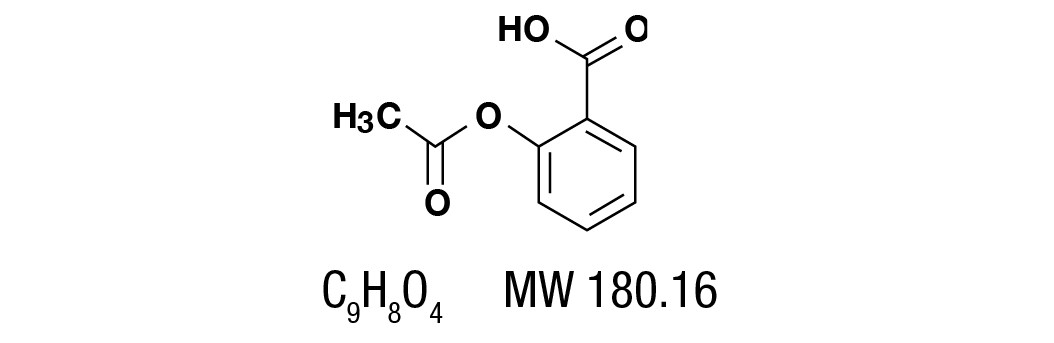
Aspirin, salicylic acid acetate, is a non-opiate analgesic, anti-inflammatory and antipyretic agent. It occurs as a white, crystalline tabular or needle like powder and is odorless or has a faint odor. It is sparingly soluble in water, freely soluble in alcohol and chloroform.
It has the following structural formula:
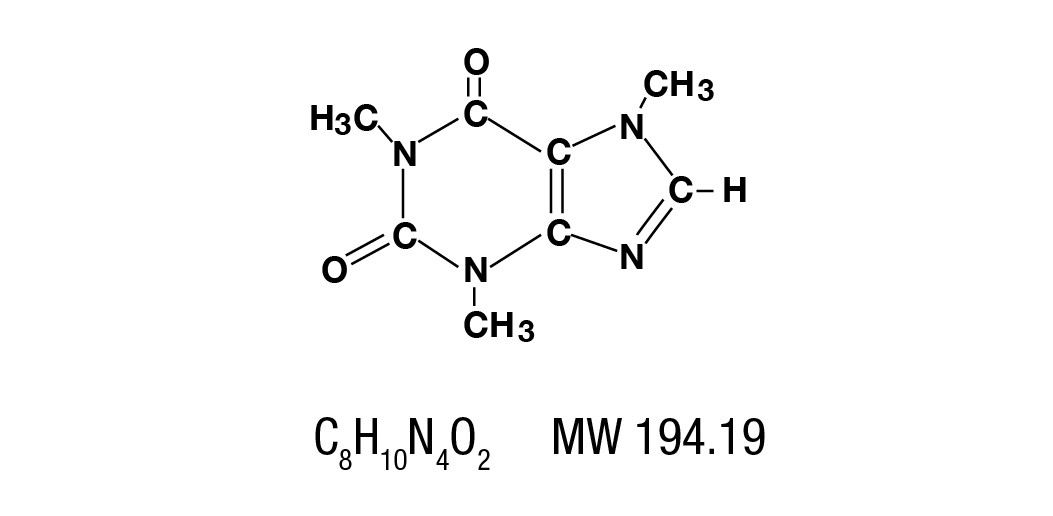
Caffeine is a central nervous system stimulant which occurs as a white powder or white glistening needles, usually matted together. It is sparingly soluble in alcohol, and freely soluble in chloroform. The chemical name for caffeine is 1,3,7-Trimethylxanthine.
It has the following structural formula:
| How Supplied |
|---|
|
Norgesic Forte Tablets (Orphenadrine Citrate 50mg, Aspirin 770mg, and Caffeine 60mg) Two-layered, white/green capsule shaped, bisected tablets debossed "GA" and "473" with bisect on the white side and plain on the green side are available in bottles of 60 tablets (NDC 50991-999-60). Manufactured for: Poly Pharmaceuticals, Inc., Owens Cross Roads, AL 35763 |
Drugs
| Drug | Countries | |
|---|---|---|
| NORGESIC FORTE | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.