STILPANE Tablet Ref.[50536] Active ingredients: Caffeine Codeine Meprobamate Paracetamol
Source: Health Products Regulatory Authority (ZA) Publisher: PHARMACARE LIMITED, Healthcare Park, Woodlands Drive, Woodmead 2191
4.1. Therapeutic indications
STILPANE is indicated for short term use (no longer than 5 days) in mild to moderate pain associated with anxiety or tension.
4.2. Posology and method of administration
For short term use only. Do not use STILPANE for longer than 5 days.
Not recommended for children under 12 years of age.
Take two tablets three or four times a day as required.
DO NOT EXCEED THE RECOMMENDED DOSE.
4.9. Overdose
Prompt treatment is essential. In the event of overdosage, consult a doctor immediately, or take the patient to the nearest hospital immediately. A delay in starting treatment may mean that antidote is given too late to be effective. Evidence of liver damage is often delayed until after the time for effective treatment has lapsed.
Susceptibility to paracetamol toxicity is increased in patients who have taken repeated high doses (greater than 5 to 10 g/day) of paracetamol for several days, in chronic alcoholism, chronic liver disease, AIDS, malnutrition, and with the use of medicines that induce liver microsomal oxidation such as barbiturates, isoniazid, rifampicin, phenytoin and carbamazepine.
Symptoms of paracetamol overdosage in the first 24 hours are pallor, nausea, vomiting, anorexia and possibly abdominal pain.
Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. Acute renal failure with acute tubular necrosis may develop even in the absence of liver damage.
Cardiac dysrhythmias have been reported.
Symptoms during the first two days of acute poisoning do not reflect the potential seriousness of the overdosage. Liver damage may manifest initially by elevation of the serum transaminase and lactic dehydrogenase activity, increased serum bilirubin concentration and prolongation of the prothrombin time. Liver damage may progress to encephalopathy, coma and death. Central oedema and non-specific myocardial depression have also occurred.
Treatment of paracetamol overdosage
Although evidence is limited it is recommended that any adult person who has ingested 5 to 10 grams or more of paracetamol (or a child who has had more than 140 mg/kg) within the preceding 4 hours, should undergo gastric lavage (emesis may be adequate for children) and a single dose of 50 g activated charcoal given via the lavage tube. Ingestion of amounts of paracetamol smaller than this may require treatment in patients susceptible to paracetamol poisoning (see above). In patients who are stuperose or comatose, endotracheal intubations should precede gastric lavage in order to avoid aspiration.
N-acetylcysteine should be administered to all cases of suspected overdose as soon as possible, preferably within eight hours of overdosage, although treatment up to 36 hours after ingestion may still be of benefit, especially if more than 150 mg/kg of paracetamol was taken.
IV: An initial dose of 150 mg/kg in 200 ml glucose injection, given intravenously over 15 minutes, followed by an infusion of 50 mg/kg in 500 ml glucose injection over the next 4 hours, and then 100 mg/kg in 1000 ml dextrose injection over the next 16 hours.
The volume of intravenous fluid should be modified for children.
Orally: Although the oral formulation is not the treatment of choice, 140 mg/kg dissolved in water as a 5% solution may be administered initially, followed by 70 mg/kg every 4 hours for 17 doses. Acetylcysteine is effective if administered preferably within 8 hours of overdosage.
If N-acetylcysteine is not available, methionine 2,5 mg may be given immediately, followed by 2,5 g every 4 hours for 3 doses. Patients should however, preferably be transferred to a facility where N-acetylcysteine can be given.
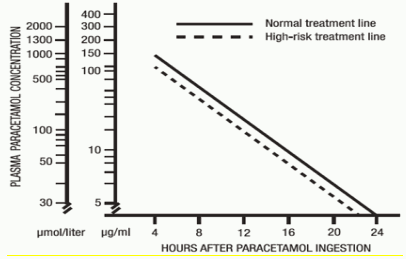
A plasma paracetamol level should be determined 4 hours after ingestion in all cases of suspected overdosage. Levels done before 4 hours, unless high may be misleading. Patients at risk of liver damage, and hence requiring continued treatment with N-acetylcysteine, can be identified according to their plasma paracetamol level. The plasma paracetamol level can be plotted against time since ingestion in the nomogram below.
Those, whose plasma paracetamol levels are above the “normal treatment line”, should continue N-acetylcysteine treatment with 100 mg/kg IV over 16 hours repeatedly until recovery. Patients with increased susceptibility to liver damage as identified above, should continue treatment if concentrations are above the “high-risk treatment line”. INR correlates best with survival. All patients with significant ingestions should be monitored for at least 96 hours.
Other symptoms include central stimulation and exhilaration, followed by cardiovascular collapse and coma.
Overdosage with barbiturates can cause severe or even fatal hypotension, respiratory depression, shock, heart failure and ultimately death (see WARNINGS AND SPECIAL PRECAUTIONS of Paracetamol).
Patients should be managed with intensive symptomatic and supportive therapy with particular attention being made to the maintenance of cardiovascular, respiratory, renal function and to the maintenance of electrolyte balance.
6.4. Special precautions for storage
Store at or below 25°C, in a well-closed container.
Protect from light.
Keep in original packaging until required for use.
KEEP OUT OF REACH OF CHILDREN.
6.5. Nature and contents of container
100 tablets are packed in aluminium/PVC blister strips. The strips are packed, together with a leaflet into a unit carton.
500 tablets are packed into a white polypropylene (PP) securitainer, together with a foam insert and a leaflet and sealed with a white polyethylene cap.
1000 tablets are packed into amber PVC jars together with a foam insert and a leaflet and sealed with a screwcap.
Not all packs and pack sizes are necessarily marketed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.